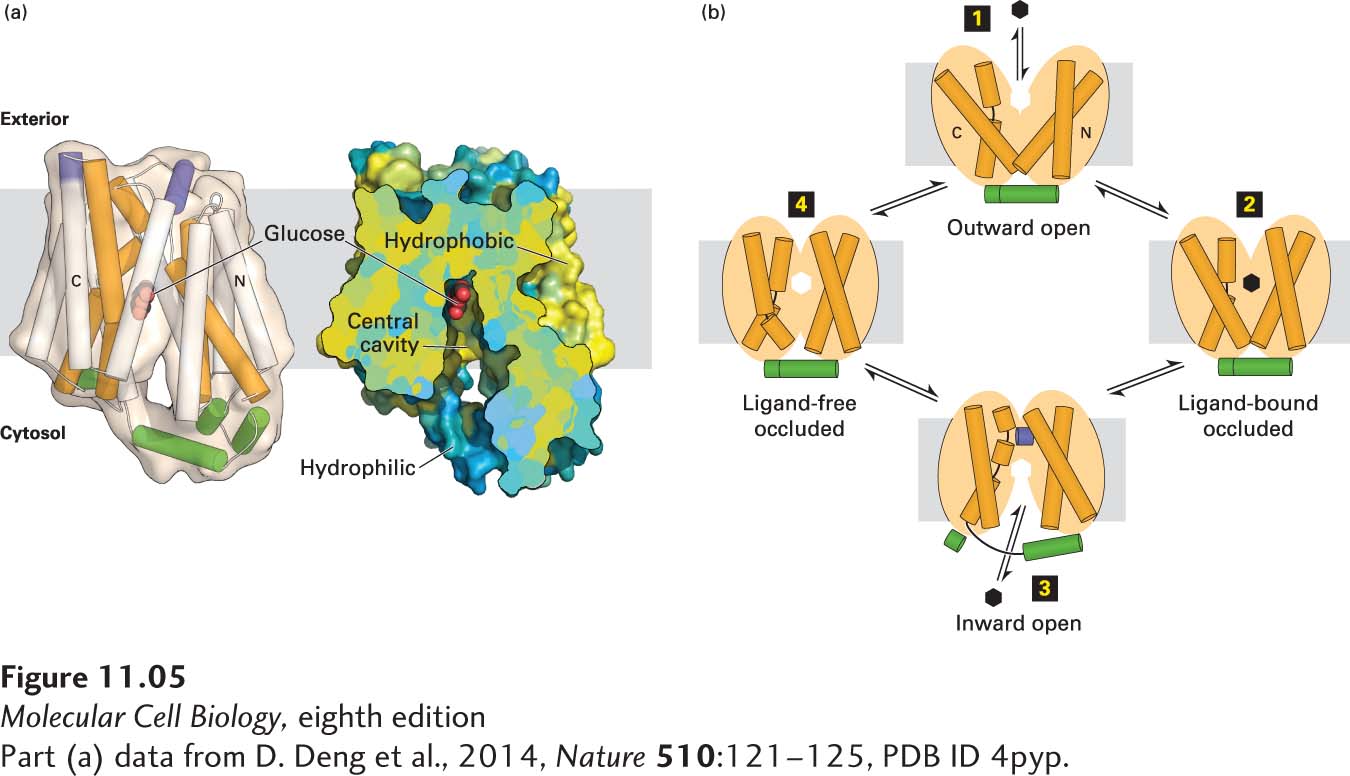
FIGURE 11- l- d- o- y- e- o- y- t- d- d- d- d-
[Part (a) data from D. Deng et al., 2014, Nature 510:121–