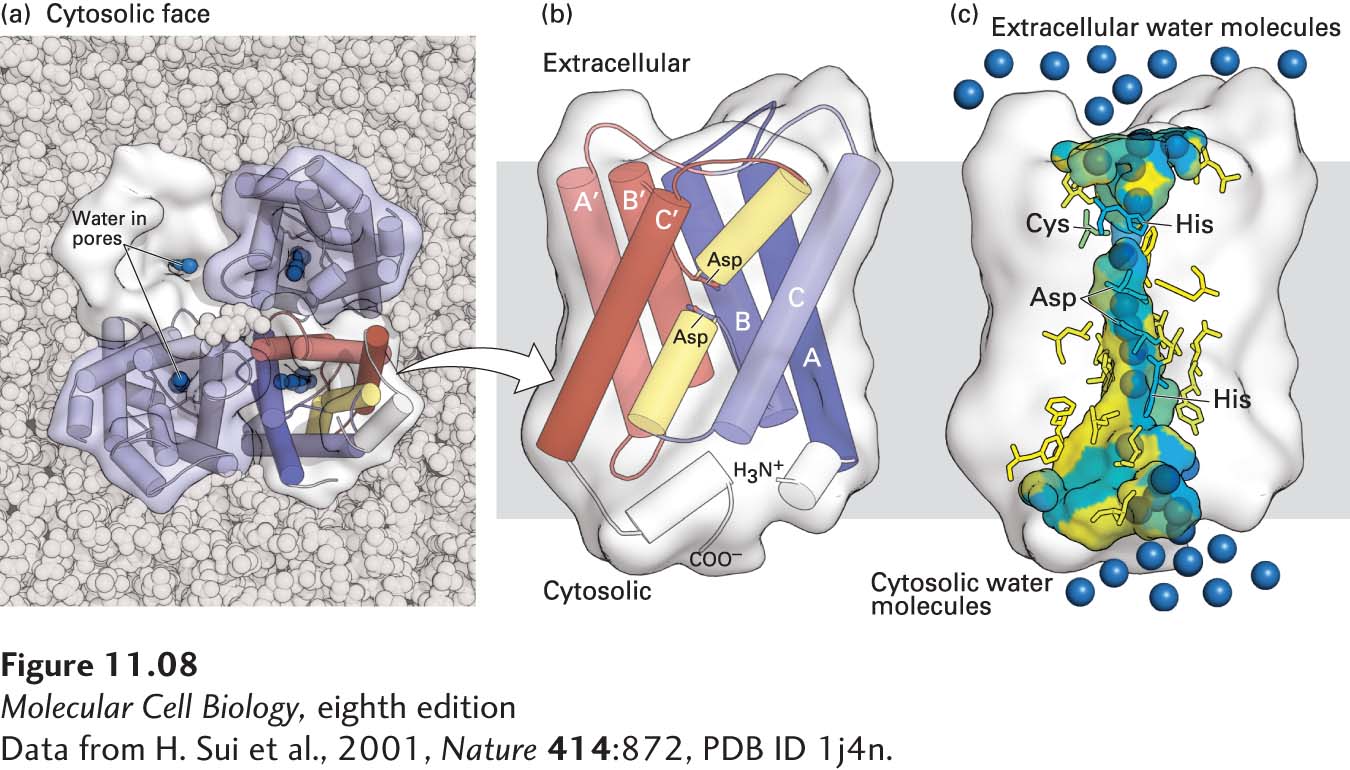
FIGURE 11- 8 Structure of an aquaporin. (a) Structural model of the tetrameric protein comprising four identical subunits. Each subunit forms a water channel, as seen in this view looking down on the protein from the exoplasmic side. One of the monomers is shown as a water- accessible surface model, in which the pore entrance can be seen. (b) Schematic diagram of the topology of a single aquaporin subunit in relation to the membrane. Three pairs of homologous transmembrane α helices (A and A′, B and B′, and C and C′) are oriented in the opposite direction with respect to the membrane and are connected by two hydrophilic loops containing short non- membrane- spanning helices and conserved asparagine (N) residues. The loops bend into the cavity formed by the six transmembrane helices, meeting in the middle to form part of the water- selective gate. (c) Side view of the pore in a single aquaporin subunit, in which several water molecules (blue spheres) are seen within the 2- nm- long water- selective gate that separates the water- filled cytosolic and extracellular vestibules. The gate contains highly conserved hydrophilic amino acid residues whose side chains form hydrogen bonds with transported water molecules. The amino acids lining the pore are colored from hydrophilic (blue) to hydrophobic (yellow). The arrangement of these hydrogen bonds and the narrow pore diameter of 0.28 nm prevent passage of protons (i.e., H3O+) or other ions. See T. Zeuthen, 2001, Trends Biochem. Sci. 26:77, and K. Murata et al., 2000, Nature 407:599.
[Data from H. Sui et al., 2001, Nature 414:872, PDB ID 1j4n.]
[Leave] [Close]