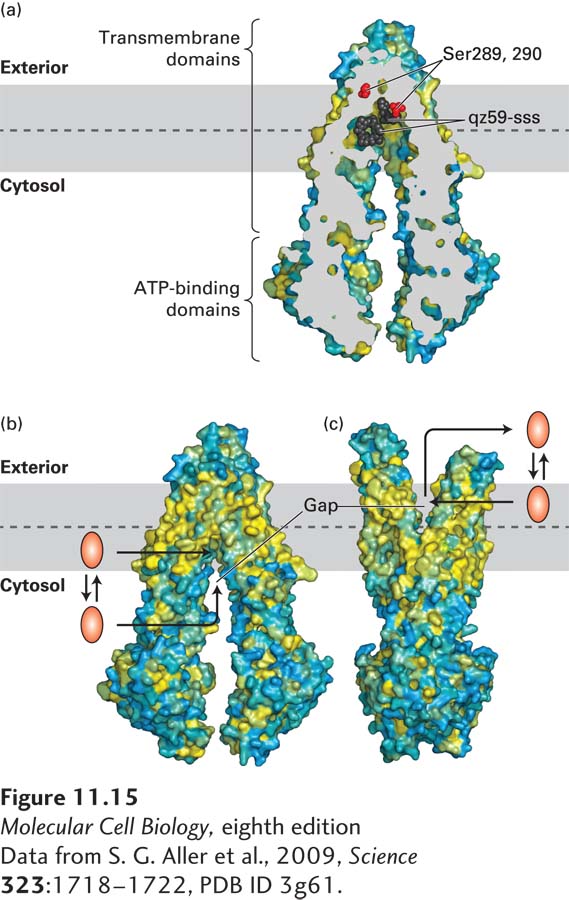
FIGURE 11- 15 The multidrug transporter ABCB1 (MDR1): Structure and model of ligand export. (a) Cross- sectional view through the center of an ABCB1 protein bound to two molecules of a drug analog, qz59- sss (black), reveals the central location of the ligand- binding site in relation to the phospholipid bilayer: the central ligand- binding cavity is close to the leaflet- leaflet interface of the membrane. During transport, this binding cavity is alternately exposed to the exoplasmic and the cytosolic surface of the membrane. Serines 289 and 290 affect the ligand specificity of the transporter; they are shown as red spheres to highlight their juxtaposition to the bound ligand. Surface residues are colored yellow to denote hydrophobic and blue to denote hydrophilic amino acids. (b) Three- dimensional structure of ABCB1 with its ligand- binding site facing inward toward the cytosol. In this conformation, a hydrophilic ligand can bind directly from the cytosol. A more hydrophobic ligand can partition into the inner leaflet of the plasma membrane bilayer and then enter the ligand- binding site through a gap in the protein that is accessible directly from the hydrophobic core of the inner leaflet. (c) Model for the structure of ABCB1 with its ligand- binding site facing outward, based on the structures of homologous bacterial ABC proteins. When the protein assumes this conformation, the ligand can either diffuse into the exoplasmic leaflet or directly into the aqueous extracellular medium. See D. Gutman et al., 2009, Trends Biochem. Sci. 35:36– 42.
[Data from S. G. Aller et al., 2009, Science 323:1718– 1722, PDB ID 3g61.]
[Leave] [Close]