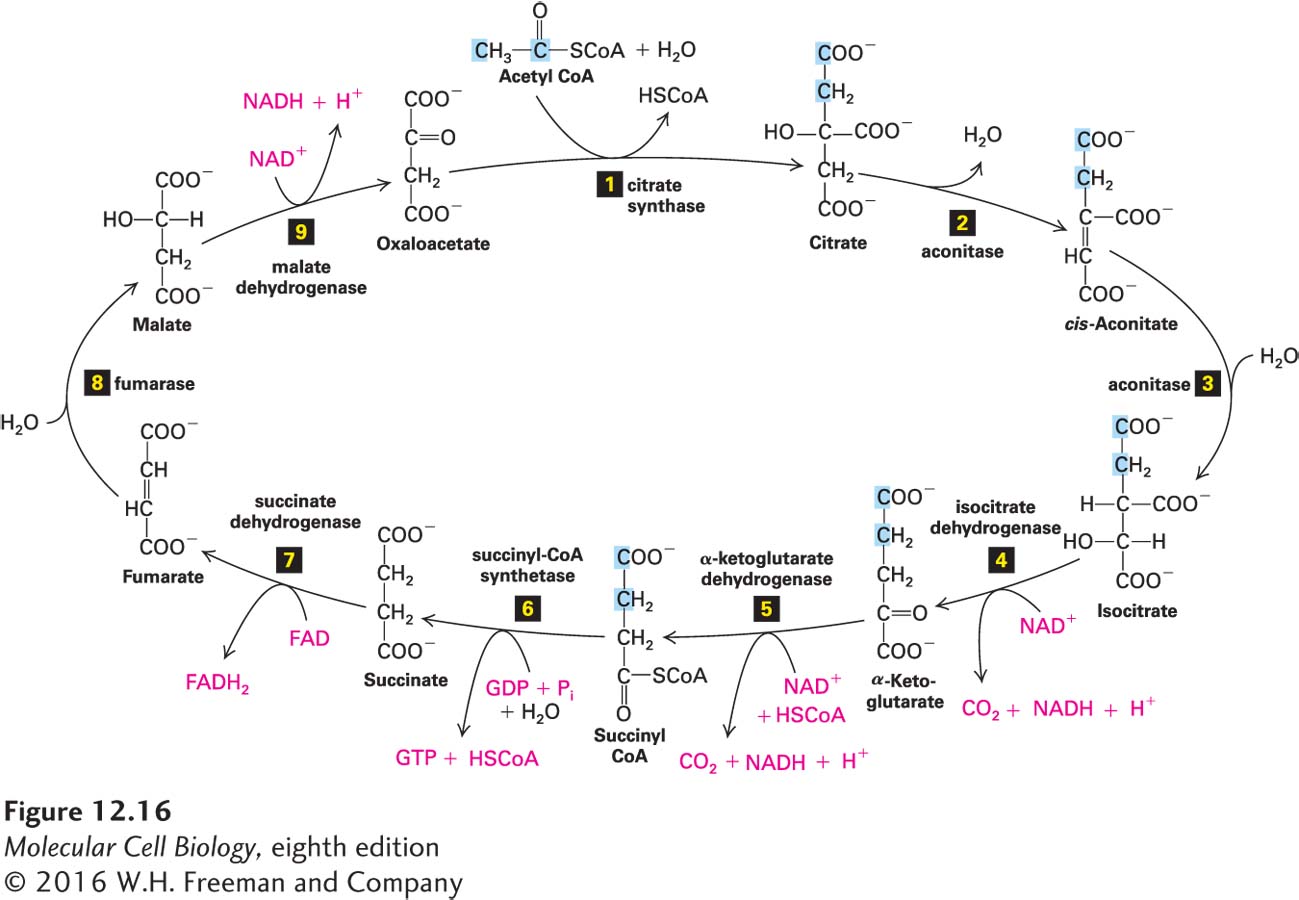
FIGURE 12- h- o- r- x- e-