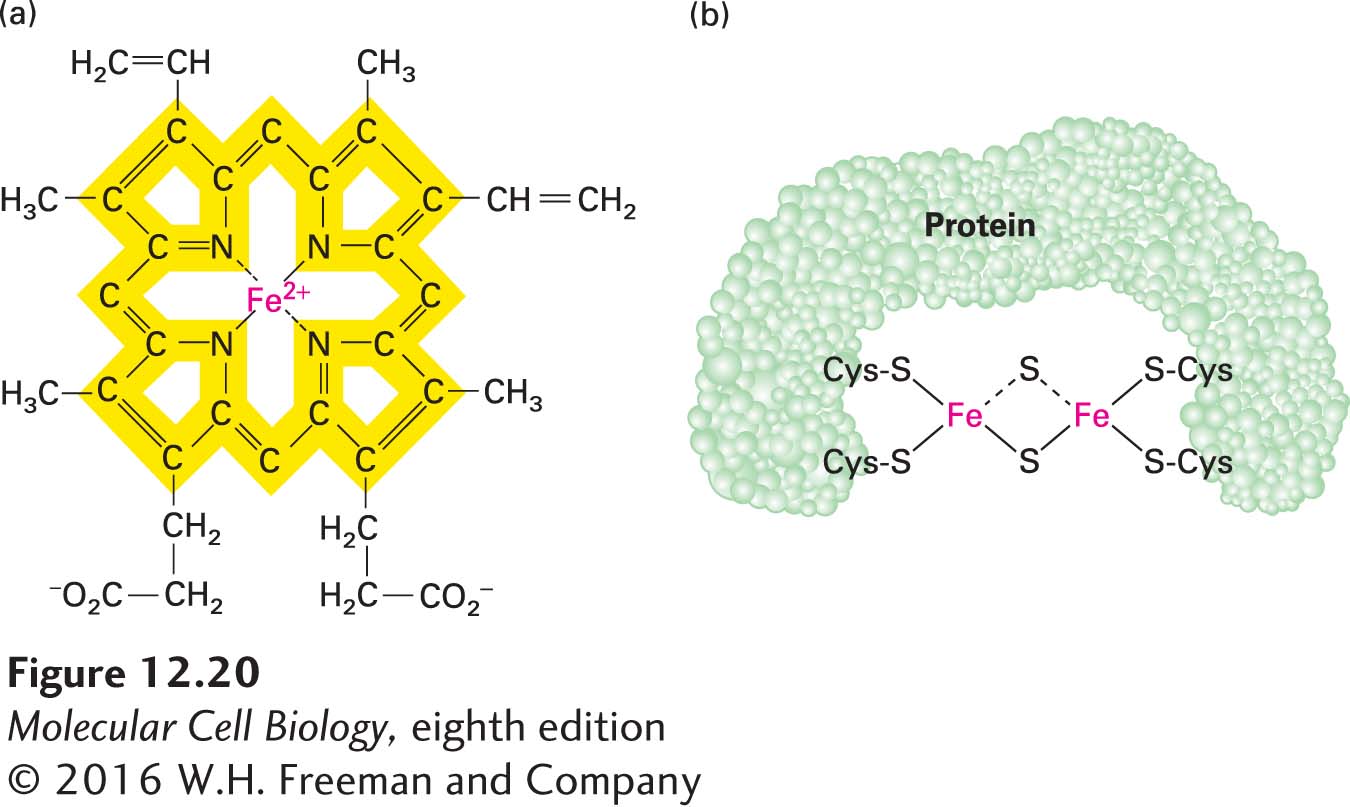
FIGURE 12- 20 Heme and iron- sulfur prosthetic groups in the electron- transport chain. (a) Heme portion of cytochromes bL and bH, which are components of CoQH2–cytochrome c reductase (complex III). The same porphyrin ring (yellow) is present in all hemes. The chemical substituents attached to the porphyrin ring differ in the other cytochromes in the electron- transport chain. All hemes accept and release one electron at a time. (b) Dimeric iron- sulfur cluster (Fe- S). Each Fe atom is bonded to four S atoms: two are inorganic sulfur, and two are in cysteine side chains of the associated protein. All Fe- S clusters accept and release one electron at a time.
[Leave] [Close]