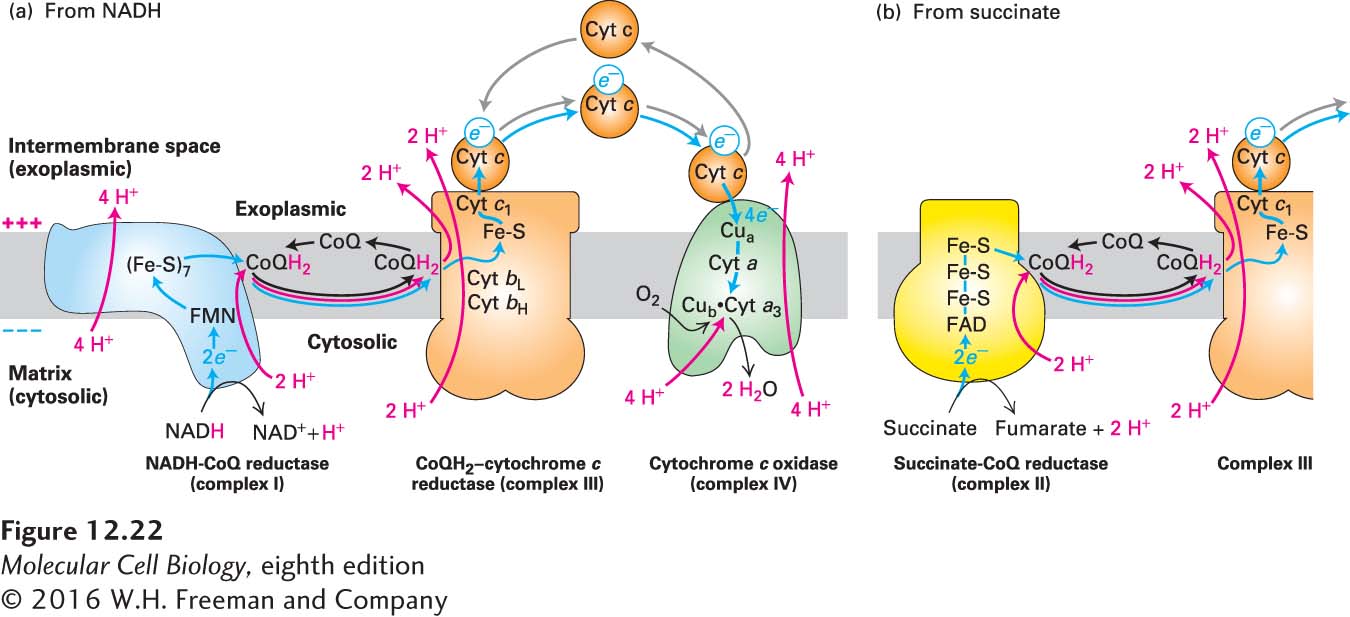
FIGURE 12- n- I– d- r- n- e- r- n- e- Q-