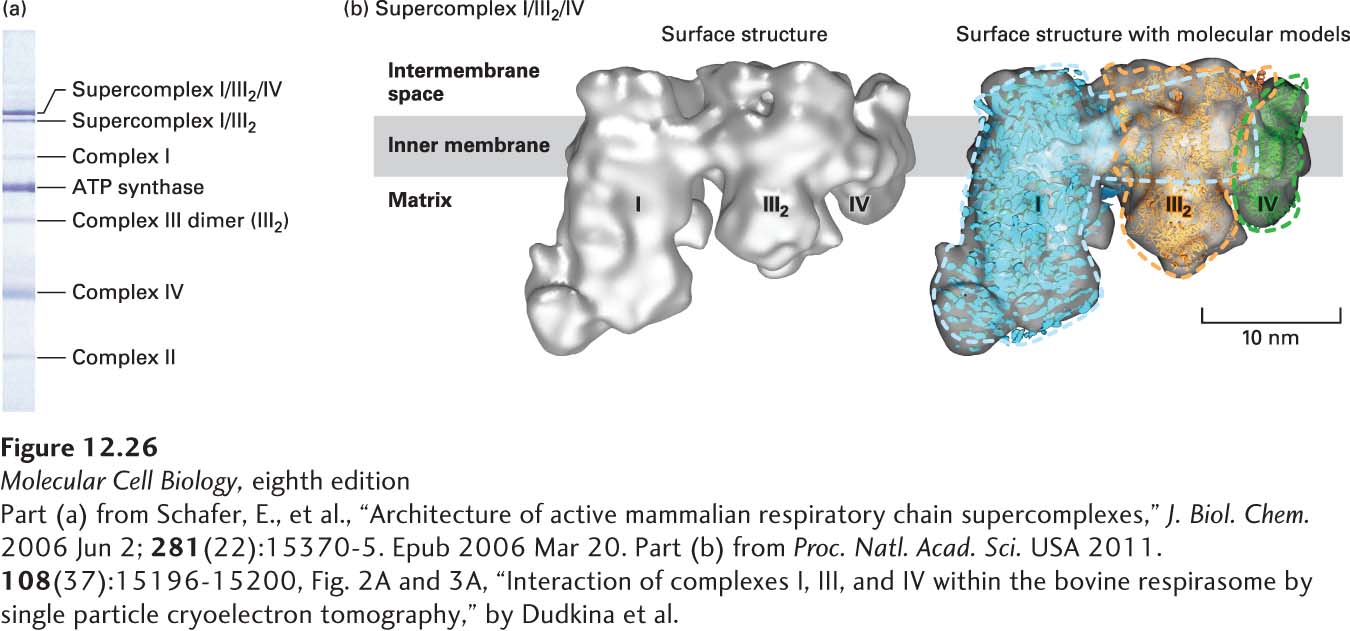
EXPERIMENTAL FIGURE 12- n- e- N- e-
[Part (a) from Schafer, E., et al., “Architecture of active mammalian respiratory chain supercomplexes,” J. Biol. Chem. 2006 Jun 2; 281(22):15370- 6-