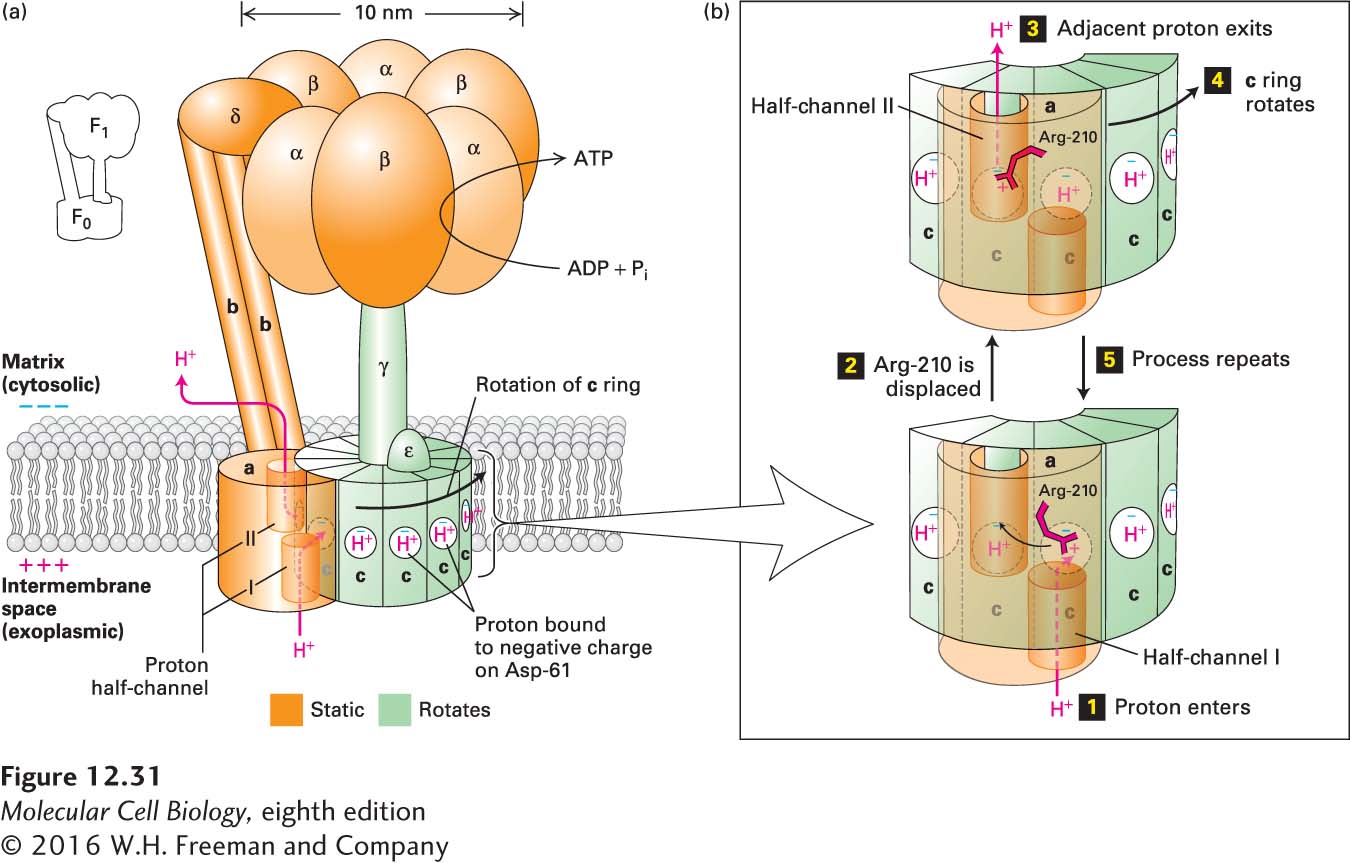
FIGURE 12- e- f- f- p- n- p- f- p- 2- d- f- p- n- p- g- n- g- n- f- n- p- f-