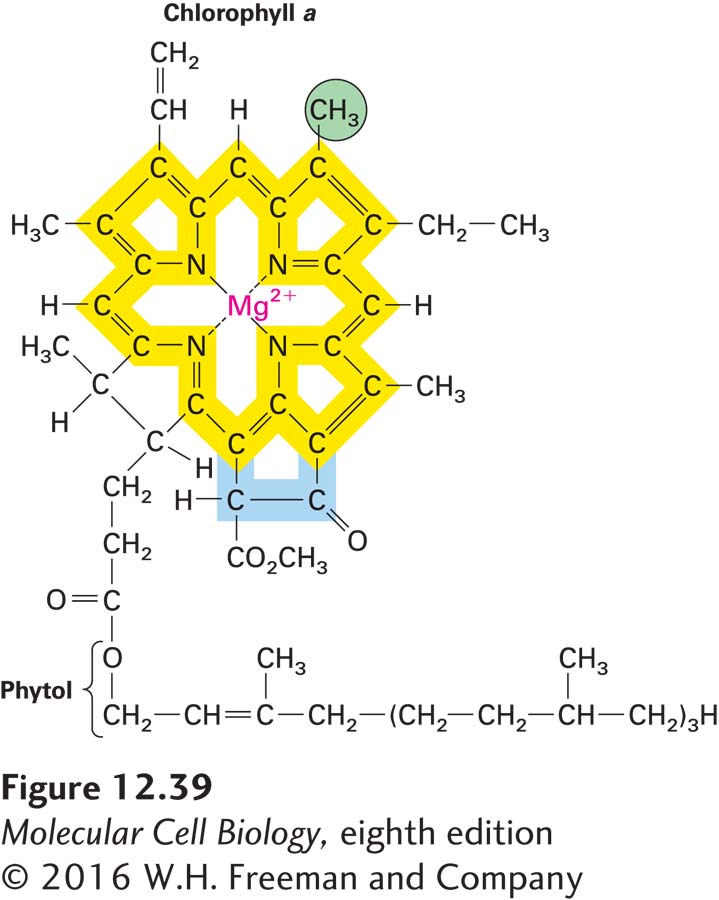
FIGURE 12- e- 2- l-