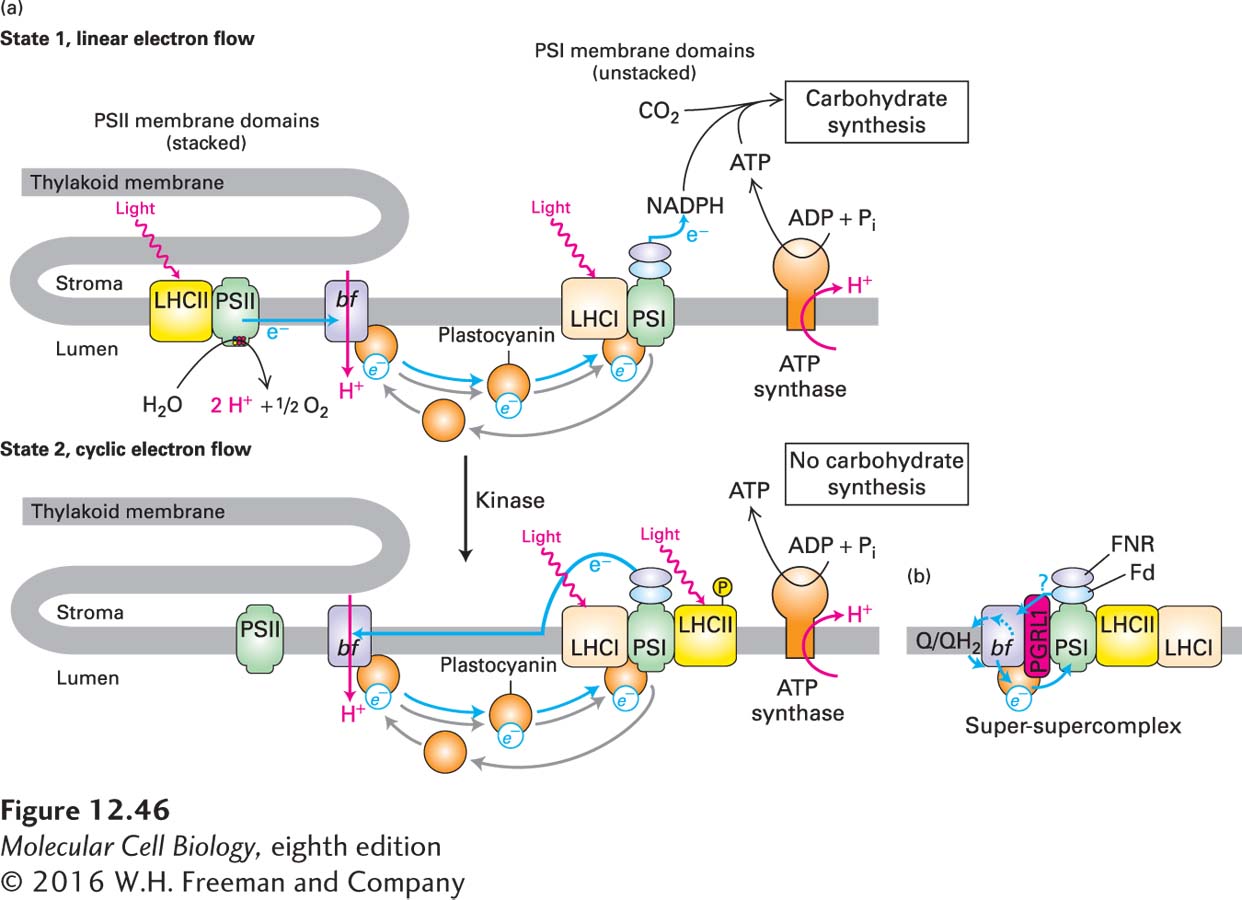
FIGURE 12- t- t- r- 1- r-