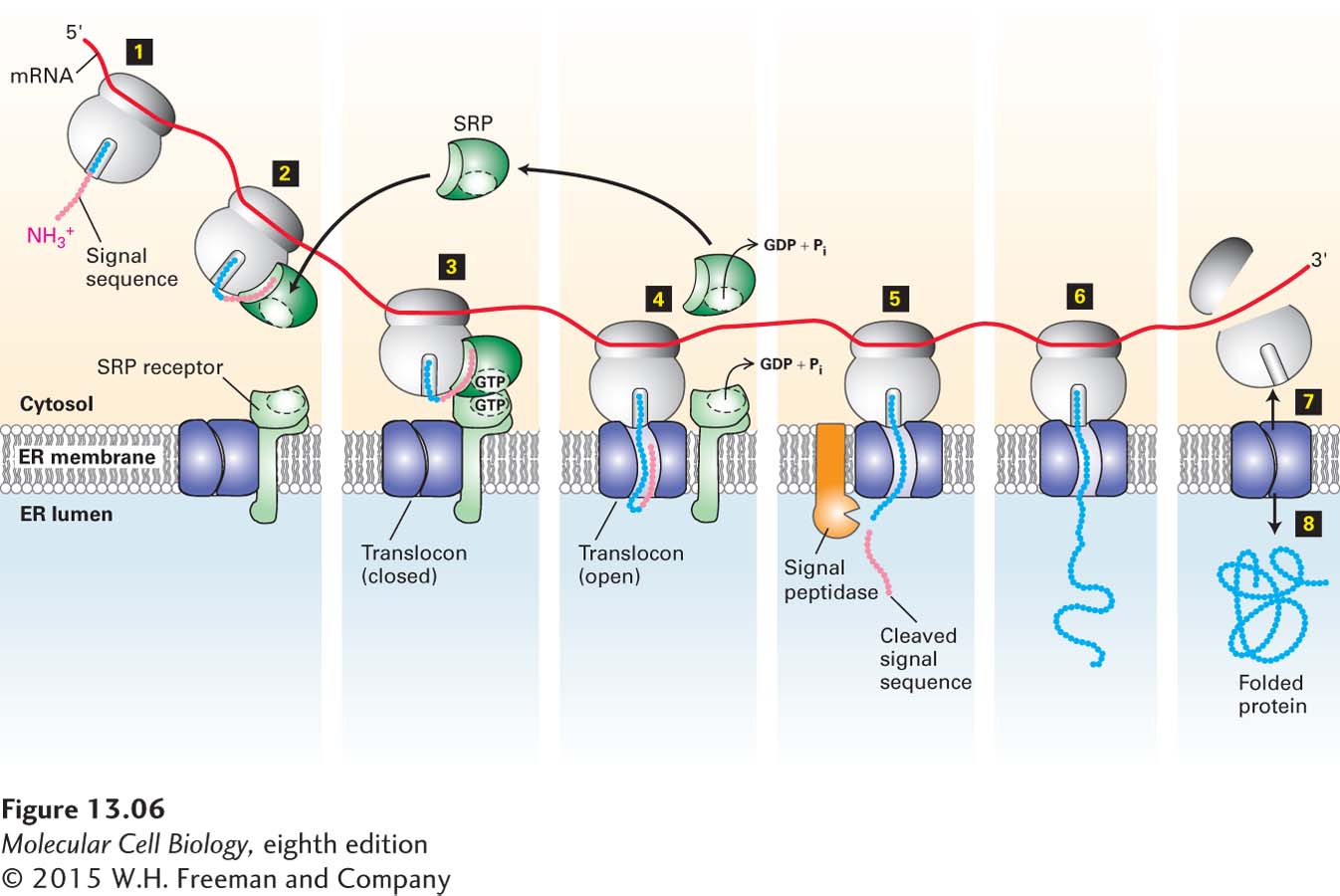
FIGURE 13- 6 Cotranslational translocation. Steps 1–2: Once the ER signal sequence emerges from the ribosome, it is bound by a signal recognition particle (SRP). Step 3: The SRP and the nascent polypeptide chain– ribosome complex bind to the SRP receptor in the ER membrane. This interaction is strengthened by the binding of GTP to both the SRP and its receptor. Step 4: Transfer of the nascent polypeptide– ribosome to the translocon leads to opening of this translocation channel to admit the growing polypeptide adjacent to the signal sequence, which is transferred to a hydrophobic binding site next to the central pore. Both the SRP and SRP receptor, once dissociated from the translocon, hydrolyze their bound GTP and then are ready to initiate the insertion of another polypeptide chain. Step 5: As the polypeptide chain elongates, it passes through the translocon channel into the ER lumen, where the signal sequence is cleaved by signal peptidase and is rapidly degraded. Step 6: The peptide chain continues to elongate as the mRNA is translated toward the 3′ end. Because the ribosome is attached to the translocon, the growing chain is extruded through the translocon into the ER lumen. Steps 7–8: Once translation is complete, the ribosome is released, the remainder of the protein is drawn into the ER lumen, the translocon closes, and the protein assumes its native folded conformation.
[Leave] [Close]