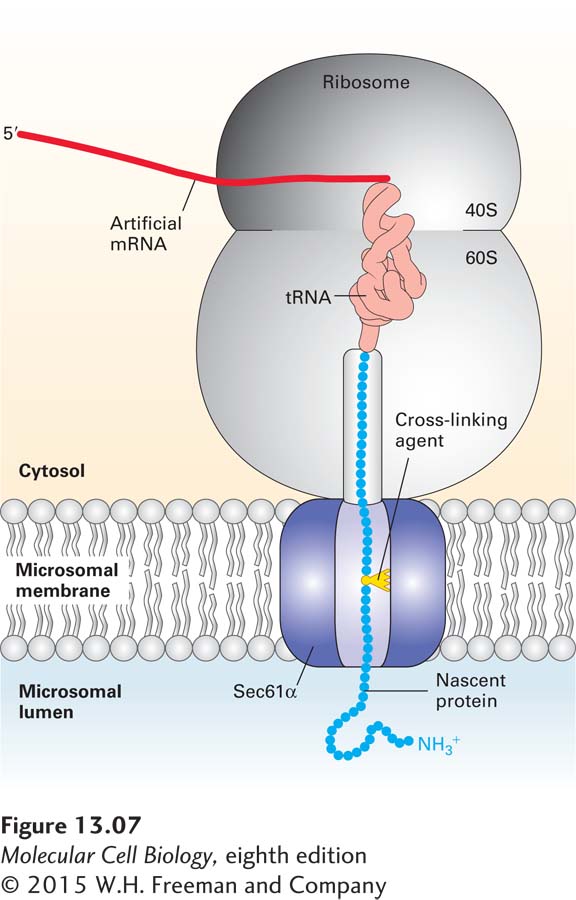
FIGURE 13- 7 Sec61α is a translocon component. Cross- linking experiments show that Sec61 α is a translocon component that contacts nascent secretory proteins as they pass into the ER lumen. An mRNA encoding the N- terminal 70 amino acids of the secreted protein prolactin was translated in a cell- free system containing microsomes (see Figure 13- 4b ). The mRNA lacked a chain- termination codon and contained one lysine codon, near the middle of the sequence. The reaction mixtures contained a chemically modified lysyl- tRNA in which a light- activated cross- linking reagent was attached to the lysine side chain. Although the entire mRNA was translated, the completed polypeptide could not be released from the ribosome without a chain- termination codon and thus became “stuck” crossing the ER membrane. The reaction mixtures were then exposed to intense light, which caused the nascent polypeptide chain to become covalently bound to whatever proteins were near it in the translocon. When the experiment was performed using microsomes from mammalian cells, the nascent chain became covalently linked to Sec61α. Different versions of the prolactin mRNA were created so that the modified lysine residue would be placed at different distances from the ribosome; cross- linking to Sec61 α was observed only when the modified lysine was positioned within the translocation channel. See T. A. Rapoport, 1992, Science 258:931 and D. Görlich and T. A. Rapoport, 1993, Cell 75:615.
[Leave] [Close]