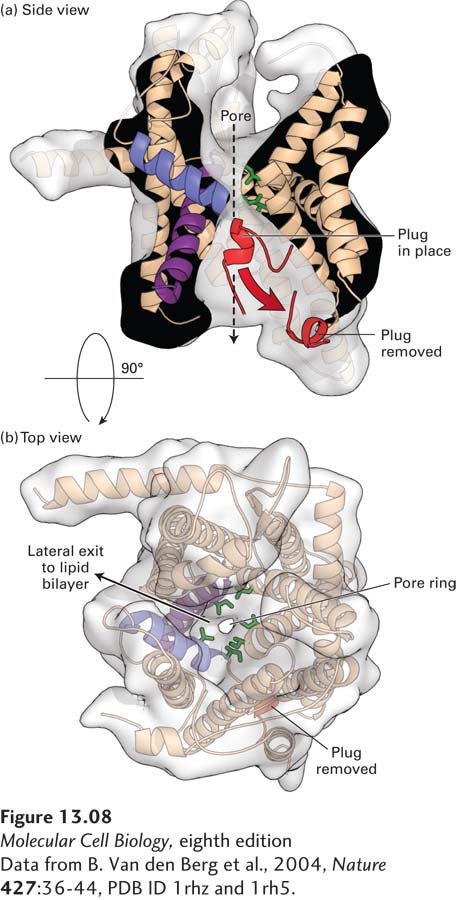
FIGURE 13- 8 Structure of an archaeal Sec61 complex. The structure of the detergent- solubilized Sec61 complex from the archaeon M. jannaschii (also known as the SecY complex) was determined by x- ray crystallography. (a) A side view shows the hourglass- shaped channel through the center of the pore. A ring of isoleucine residues at the constricted waist of the pore forms a gasket that keeps the channel sealed to small molecules even as a translocating polypeptide passes through the channel. When no translocating peptide is present, the channel is closed by a short helical plug (red). This plug moves out of the channel during translocation. In this view, the front half of protein has been removed to better show the pore. (b) A view looking through the center of the channel shows a region (on the left side) where helices may separate, allowing lateral passage of a hydrophobic transmembrane domain into the lipid bilayer.
[Data from B. Van den Berg et al., 2004, Nature 427:36– 44, PDB ID 1rhz and 1rh5.]
[Leave] [Close]