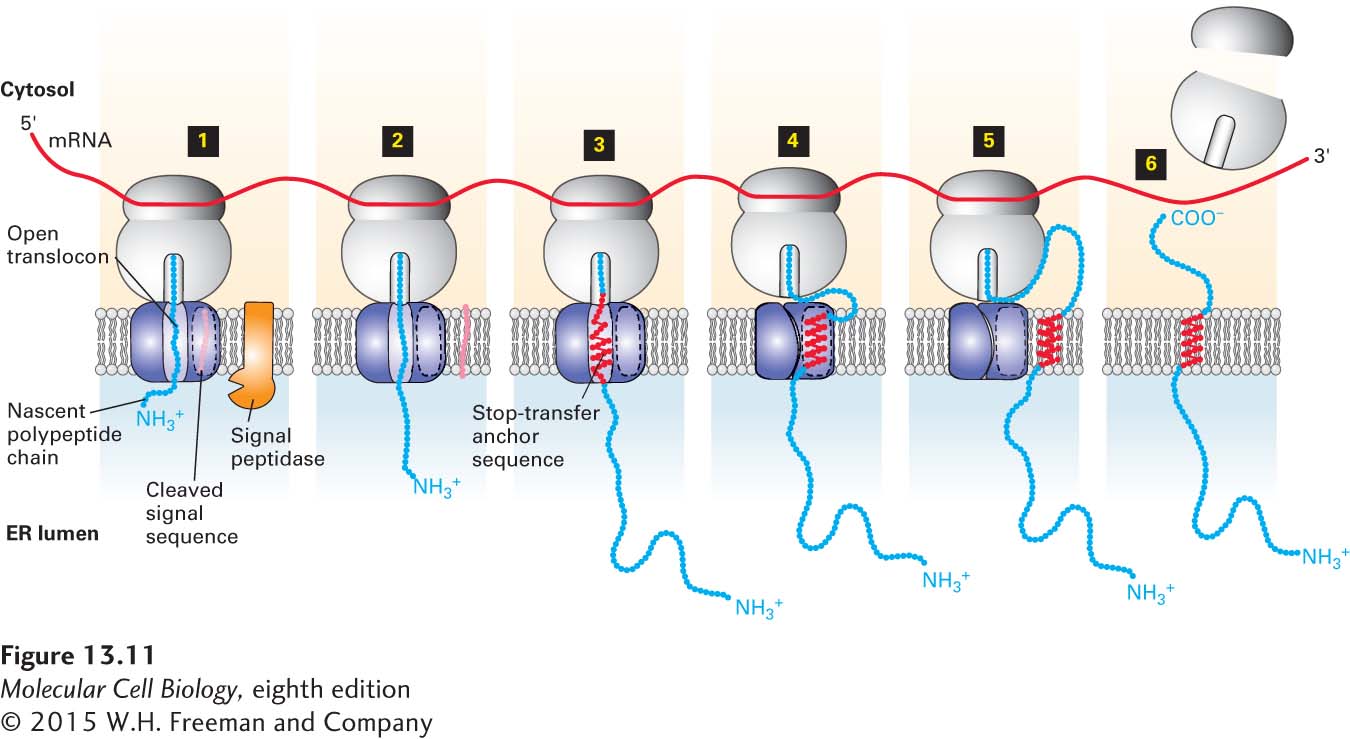
FIGURE 13- 11 Membrane insertion and orientation of type I single- pass transmembrane proteins. Step 1: After the nascent polypeptide chain– ribosome complex becomes associated with a translocon in the ER membrane, the N- terminal signal sequence is cleaved. This process occurs by the same mechanism as the one for soluble secretory proteins (see Figure 13- 6 ). Steps 2–3: The chain is elongated until the hydrophobic stop- transfer anchor sequence is synthesized and enters the translocon, where it prevents the nascent chain from extruding farther into the ER lumen. Step 4: The stop- transfer anchor sequence moves laterally through a hydrophobic cleft between translocon subunits and ultimately becomes anchored in the phospholipid bilayer. At this time, the translocon probably closes. Step 5: As synthesis continues, the elongating chain may loop out into the cytosol through the small space between the ribosome and translocon. Step 6: When synthesis is complete, the ribosomal subunits are released into the cytosol, leaving the protein free to diffuse laterally in the membrane. See H. Do et al., 1996, Cell 85:369, and W. Mothes et al., 1997, Cell 89:523.
[Leave] [Close]