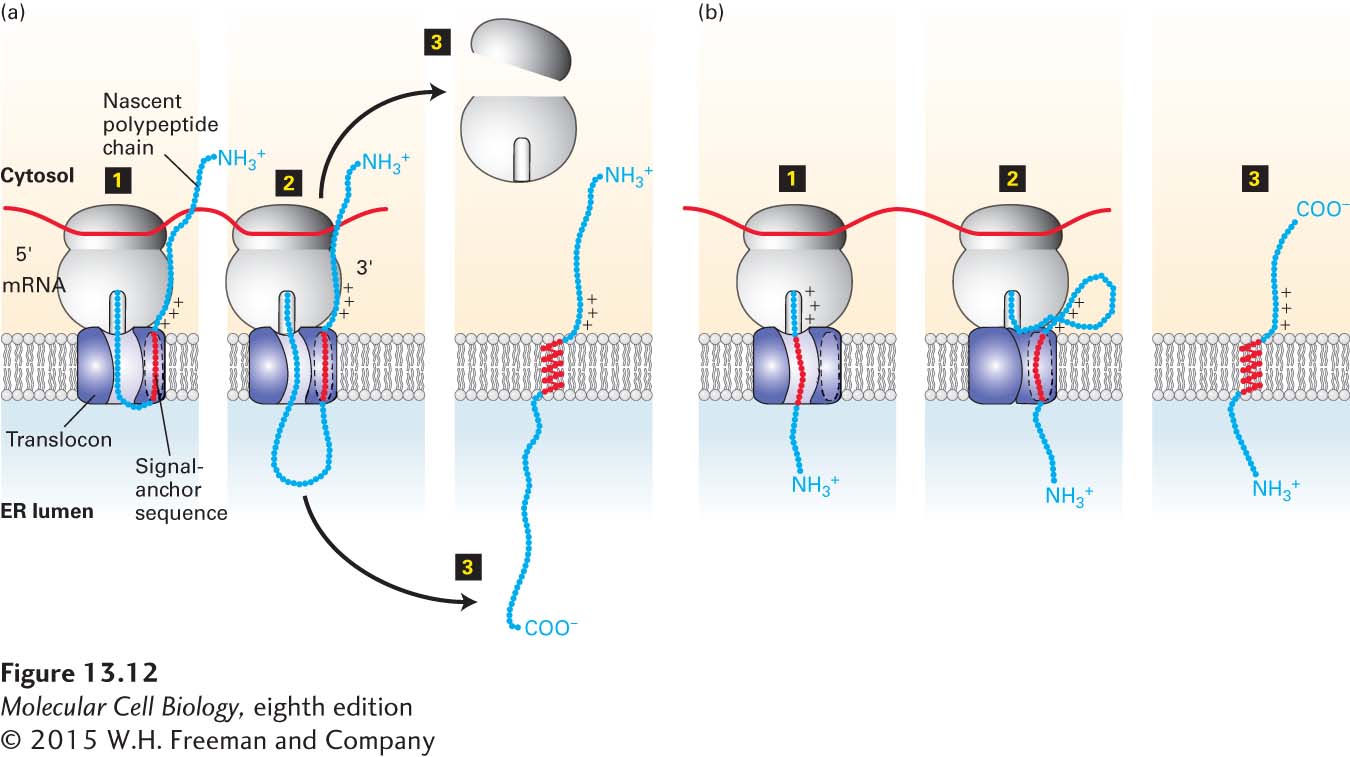
FIGURE 13- 12 Membrane insertion and orientation of type II and type III single- pass transmembrane proteins. (a) Type II proteins. Step 1: After the internal signal- anchor sequence is synthesized on a cytosolic ribosome, it is bound by an SRP (not shown), which binds the SRP receptor on the ER membrane. This process is similar to the targeting of soluble secretory proteins except that the hydrophobic signal sequence is not located at the N- terminus and is not subsequently cleaved. The nascent polypeptide chain becomes oriented in the translocon with its N- terminal portion toward the cytosol. This orientation is dictated by the positively charged residues shown N- terminal to the signal- anchor sequence. Step 2: As the chain is elongated and extruded into the lumen, the internal signal- anchor sequence moves laterally through a hydrophobic cleft between translocon subunits and anchors the chain in the phospholipid bilayer. Step 3: Once protein synthesis is complete, the C- terminus of the polypeptide is released into the lumen, and the ribosomal subunits are released into the cytosol. (b) Type III proteins. Step 1: Insertion is by a process similar to that of type II proteins, except that positively charged residues on the C- terminal side of the signal- anchor sequence cause the transmembrane segment to be oriented within the translocon with its C- terminal portion toward the cytosol and the N- terminal end in the ER lumen. Steps 2–3: Elongation of the C- terminal portion of the polypeptide chain is completed in the cytosol, and the ribosomal subunits are released. See M. Spiess and H. F. Lodish, 1986, Cell 44:177, and H. Do et al., 1996, Cell 85:369.
[Leave] [Close]