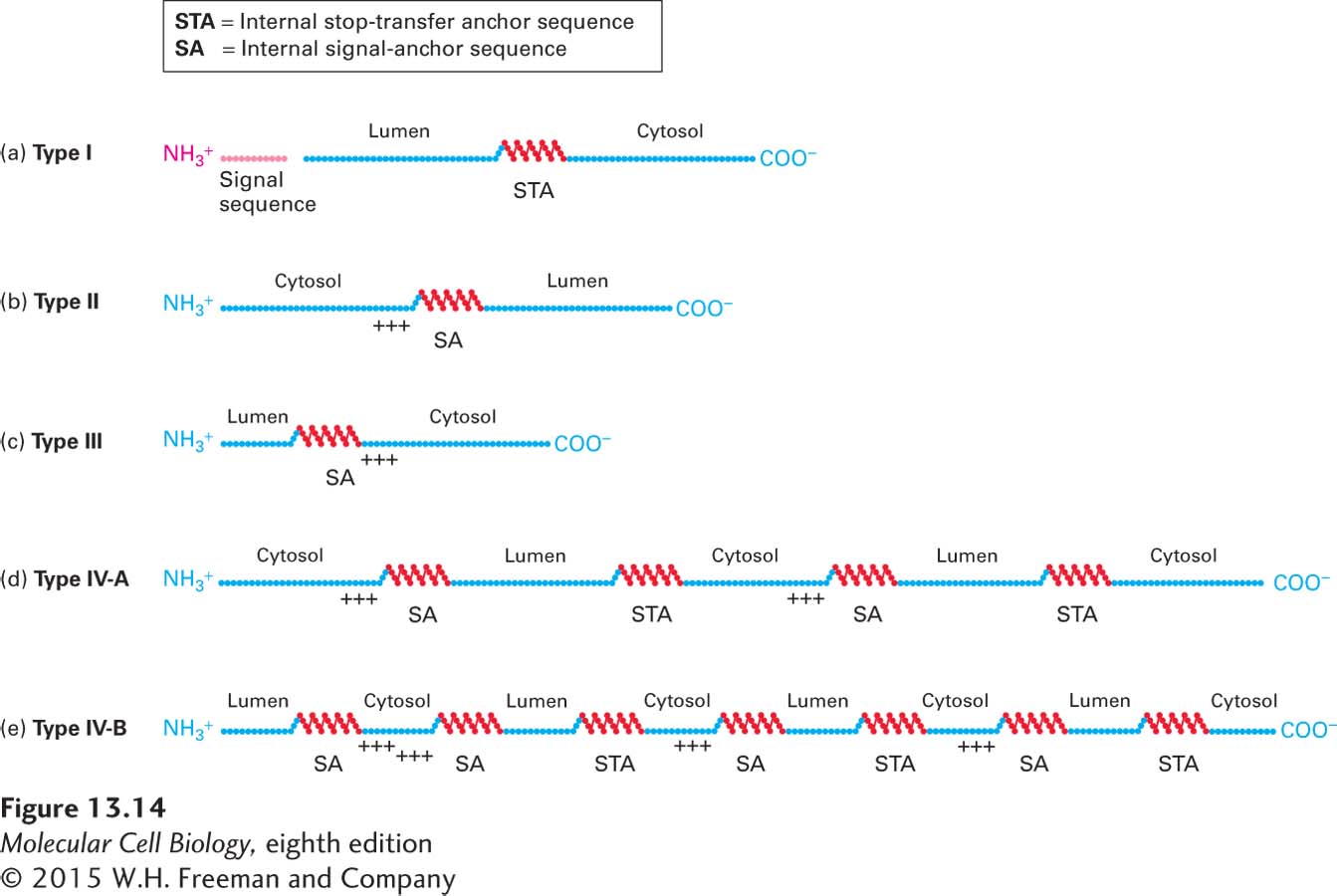
FIGURE 13- 14 Topogenic sequences determine the orientation of ER membrane proteins. Topogenic sequences are shown in red; soluble, hydrophilic sequences in blue. The internal topogenic sequences form transmembrane α helices that anchor proteins or segments of proteins in the membrane. (a) Type I proteins contain a cleaved signal sequence and a single internal stop- transfer anchor (STA) sequence. (b, c) Type II and type III proteins contain a single internal signal- anchor (SA) sequence. The difference in the orientation of these protein types depends largely on whether there is a high density of positively charged amino acids (+ + +) on the N- terminal side (type II) or on the C- terminal side of the SA sequence (type III). (d, e) Nearly all multipass proteins lack a cleavable signal sequence, as depicted in the examples shown here. Type IV- A proteins, whose N- terminus faces the cytosol, contain alternating type II SA sequences and STA sequences. Type IV- B proteins, whose N- terminus faces the lumen, begin with a type III SA sequence followed by alternating type II SA and STA sequences. Proteins of each type with different numbers of α helices (odd or even) are known.
[Leave] [Close]