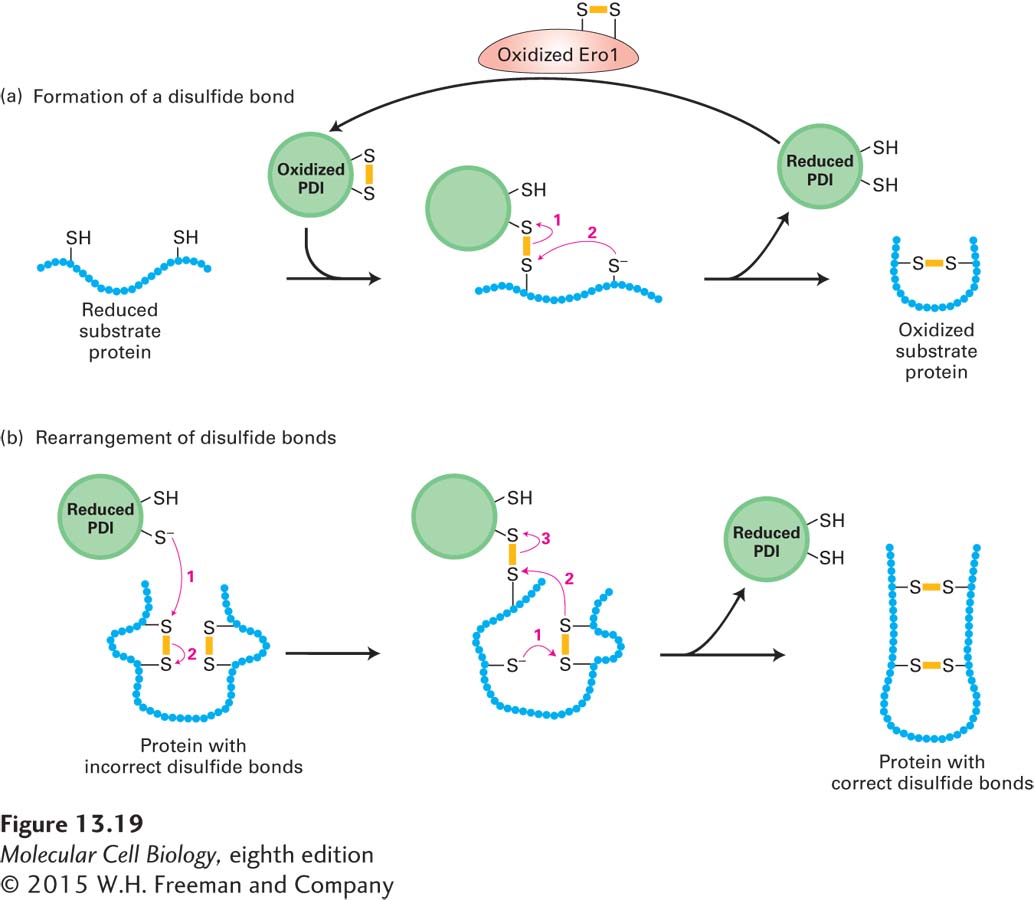
FIGURE 13- 19 Action of protein disulfide isomerase (PDI). PDI forms and rearranges disulfide bonds via an active site with two closely spaced cysteine residues that are easily interconverted between the reduced dithiol form and the oxidized disulfide form. Numbered red arrows indicate the sequence of electron transfers. Yellow bars represent disulfide bonds. (a) In the formation of disulfide bonds, the ionized (–S−) form of a cysteine thiol in the substrate protein reacts with the disulfide (S– S) bond in oxidized PDI to form a disulfide- bonded PDI– substrate protein intermediate. A second ionized thiol in the substrate protein then reacts with this intermediate, forming a disulfide bond within the substrate protein and releasing reduced PDI. PDI, in turn, transfers electrons to a disulfide bond in the luminal protein Ero1, thereby regenerating the oxidized form of PDI. (b) Reduced PDI can catalyze rearrangement of improperly formed disulfide bonds by similar thiol- disulfide transfer reactions. In this case, reduced PDI both initiates and is regenerated in the reaction pathway. These reactions are repeated until the most stable conformation of the protein is achieved. See M. M. Lyles and H. F. Gilbert, 1991, Biochemistry 30:619.
[Leave] [Close]