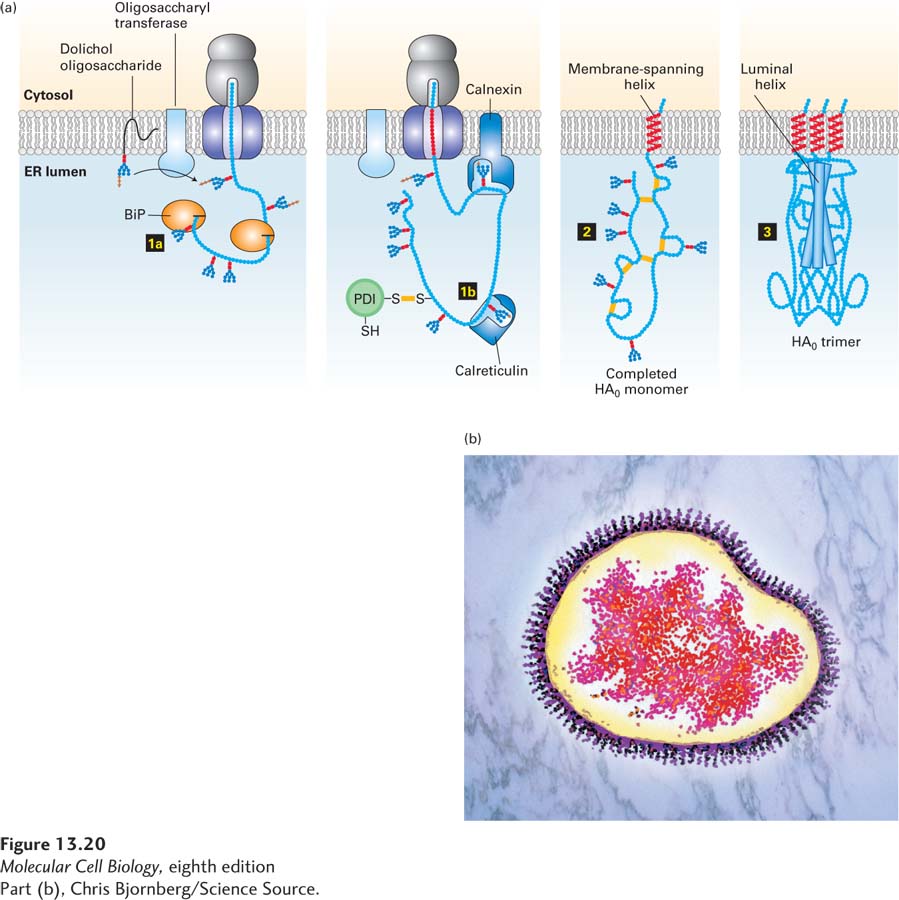
FIGURE 13- 20 Hemagglutinin folding and assembly. (a) Mechanism of (HA0) trimer assembly. Transient binding of the chaperone BiP (step 1a) to the nascent polypeptide chain and of two lectins, calnexin and calreticulin, to certain oligosaccharide chains (step 1b) promotes proper folding of adjacent segments of HA0. A total of seven N-linked oligosaccharide chains are added to the luminal portion of the nascent chain during cotranslational translocation, and PDI catalyzes the formation of six disulfide bonds per monomer. Completed HA0 monomers are anchored in the membrane by a single membrane- spanning α helix with the N- terminus in the lumen (step 2). Interaction of three HA0 chains with one another, initially via their transmembrane α helices, apparently triggers formation of a long stem containing one α helix from the luminal part of each HA0 polypeptide. Finally, interactions occur among the three globular heads, generating a stable HA0 trimer (step 3). (b) Electron micrograph (false color) of a complete influenza virion showing trimers of HA protein protruding as spikes from the surface of the viral membrane. See U. Tatu et al., 1995, EMBO J. 14:1340, and D. Hebert et al., 1997, J. Cell Biol. 139:613.
[Part (b), Chris Bjornberg/Science Source.]
[Leave] [Close]