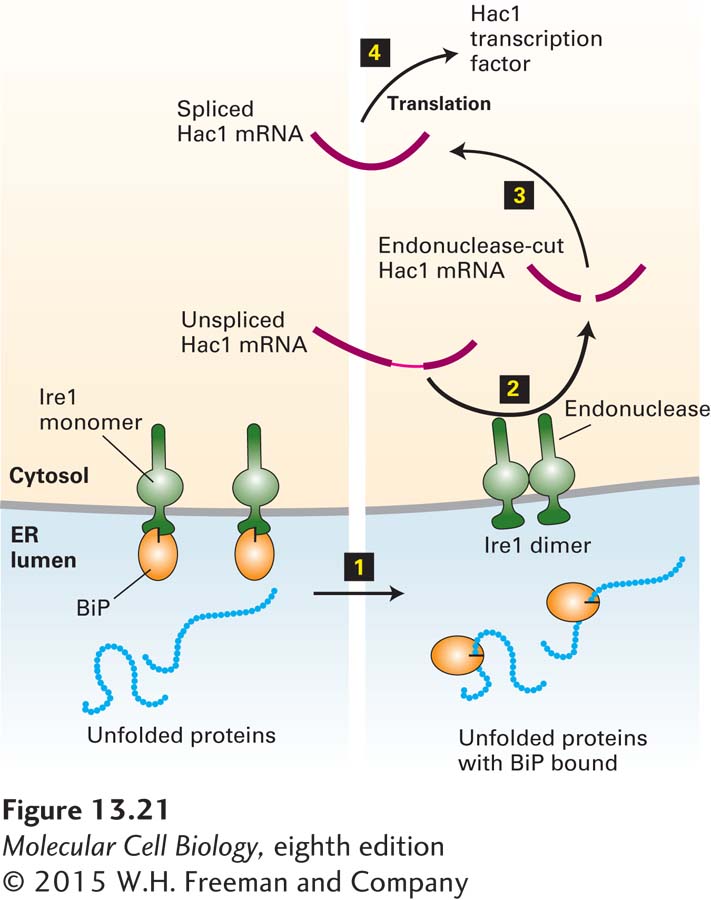
FIGURE 13- 21 The unfolded- protein response. Ire1, a transmembrane protein in the ER membrane, has a binding site for BiP on its luminal domain; the cytosolic domain contains a specific RNA endonuclease. Step 1: Accumulating unfolded proteins in the ER lumen bind BiP molecules, releasing them from monomeric Ire1. Dimerization of Ire1 then activates its endonuclease activity. Steps 2–3: The unspliced mRNA precursor encoding the transcription factor Hac1 is cleaved by dimeric Ire1, and the two exons are joined to form functional Hac1 mRNA. Current evidence indicates that this processing occurs in the cytosol, although pre- mRNA processing generally occurs in the nucleus. Step 4: Hac1 is translated into Hac1 protein, which then moves back into the nucleus and activates transcription of genes encoding several protein- folding catalysts. See U. Ruegsegger et al., 2001, Cell 107:103; A. Bertolotti et al., 2000, Nat. Cell Biol. 2:326; and C. Sidrauski and P. Walter, 1997, Cell 90:1031.
[Leave] [Close]