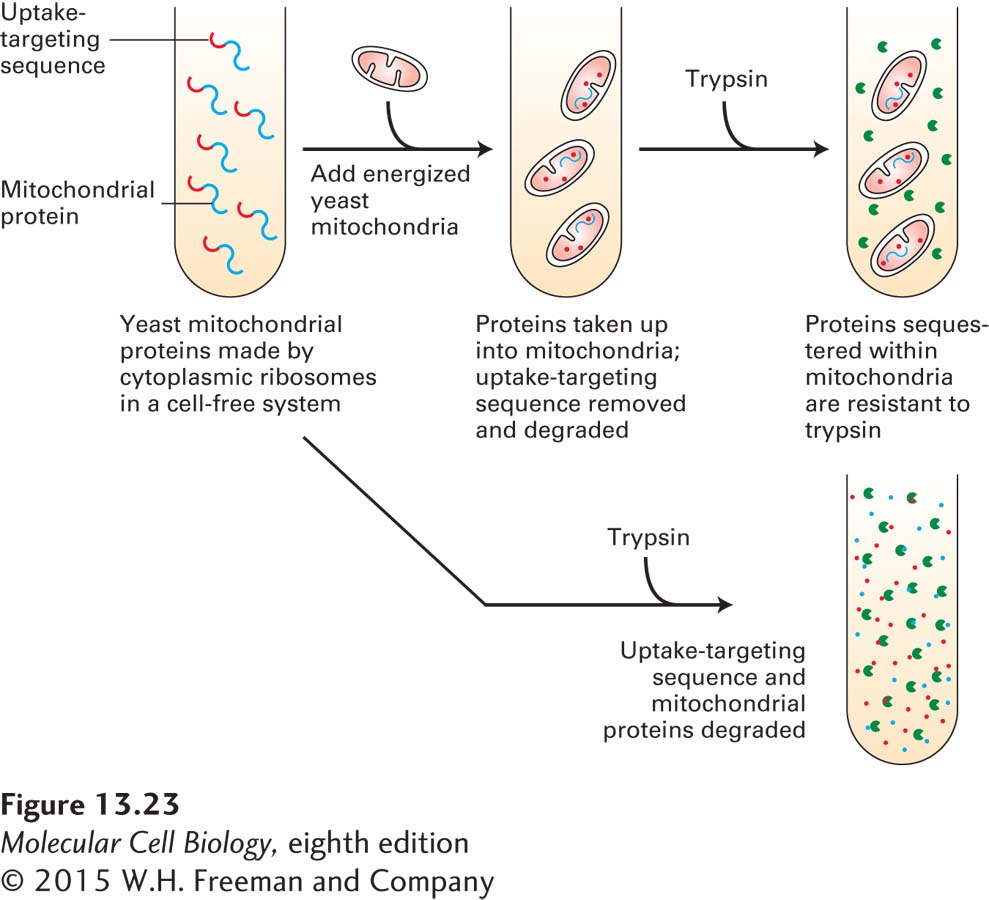
FIGURE 13- 23 Import of mitochondrial precursor proteins is assayed in a cell- free system. Mitochondrial precursor proteins with attached uptake- targeting signals can be synthesized on ribosomes in a cell- free reaction. When respiring mitochondria are added to these synthesized mitochondrial precursor proteins (top), the proteins are taken up by mitochondria. Inside mitochondria, the proteins are protected from the action of proteases such as trypsin. When no mitochondria are present (bottom), the mitochondrial proteins are degraded by added protease. Only energized (respiring) mitochondria, which have a proton electrochemical gradient (proton- motive force) across the inner membrane, take up these proteins. Furthermore, the imported proteins must contain an appropriate uptake- targeting sequence. Uptake also requires ATP and a cytosolic extract containing chaperone proteins that maintain the precursor proteins in an unfolded conformation. This assay has been used to study targeting sequences and other features of the translocation process.
[Leave] [Close]