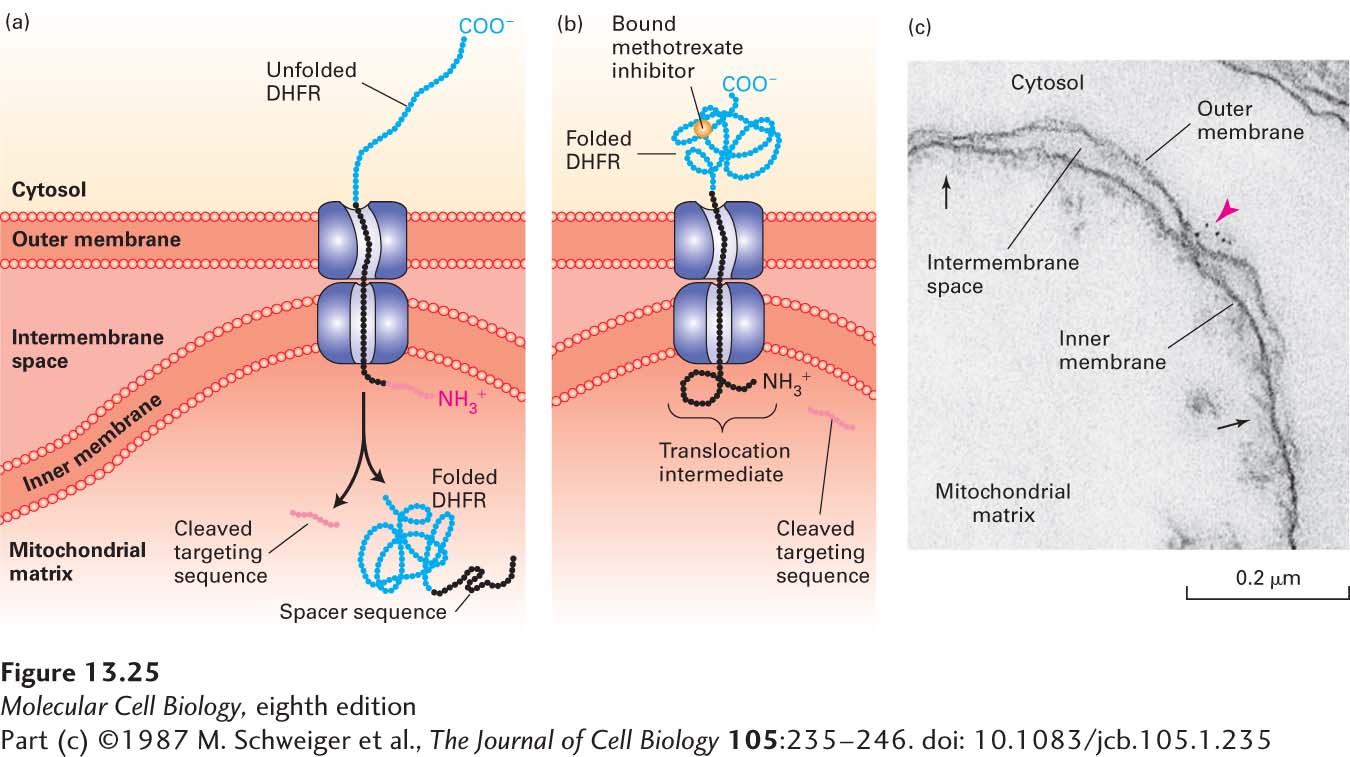
EXPERIMENTAL FIGURE 13- 25 Experiments with chimeric proteins elucidate mitochondrial protein import processes. These experiments show that a matrix- targeting sequence alone directs proteins to the mitochondrial matrix and that only unfolded proteins are translocated across both mitochondrial membranes. The chimeric protein in these experiments contained a matrix- targeting signal at its N- terminus (red), followed by a spacer sequence of no particular function (black), and then by dihydrofolate reductase (DHFR), an enzyme normally present only in the cytosol. (a) When the DHFR segment is unfolded, the chimeric protein moves across both membranes to the matrix of an energized mitochondrion, and the matrix- targeting signal is then removed. (b) When the C- terminus of the chimeric protein is locked in the folded state by binding of methotrexate, translocation is blocked. If the spacer sequence is long enough to extend across both transport channels, a stable translocation intermediate, with the targeting sequence cleaved off, is generated in the presence of methotrexate, as shown here. (c) The C- terminus of the translocation intermediate in (b) can be detected by incubating the mitochondria with antibodies that bind to the DHFR segment, followed by gold particles coated with bacterial protein A, which binds nonspecifically to antibody molecules (see Figure 4-33). An electron micrograph of a sectioned sample reveals gold particles (red arrowhead) bound to the translocation intermediate at a contact site between the inner and outer membranes. Other contact sites (black arrows) are also evident. See J. Rassow et al., 1990, FEBS Lett. 275:190.
[Part (c) ©1987 M. Schweiger et al., The Journal of Cell Biology 105:235– 246. doi: 10.1083/jcb.105.1.235]
[Leave] [Close]