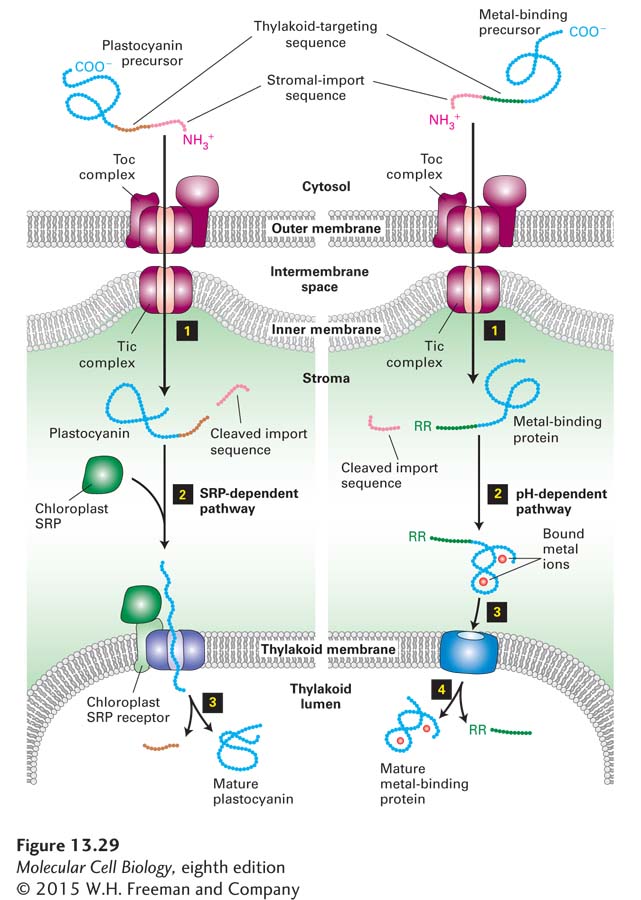
FIGURE 13- 29 Transporting proteins to chloroplast thylakoids. Two of the four pathways for transporting proteins from the cytosol to the thylakoid lumen are shown here. In these pathways, unfolded precursors are delivered to the stroma via the same outer- membrane proteins that import stromal- localized proteins. Cleavage of the N- terminal stromal- import sequence by a stromal protease then reveals the thylakoid- targeting sequence (step 1). At this point the two pathways diverge. In the SRP- dependent pathway (left), plastocyanin and similar proteins are kept unfolded in the stromal space by a set of chaperones (not shown), and the thylakoid- targeting sequence binds to proteins that are closely related to the bacterial SRP, SRP receptor, and SecY translocon, which mediate movement into the thylakoid lumen (step 2). After the thylakoid- targeting sequence is removed in the thylakoid lumen by a separate endoprotease, the protein folds into its mature conformation (step 3). In the pH- dependent pathway (right), metal- binding proteins fold in the stroma, and complex redox cofactors are added (step 2). Two arginine residues (RR) at the N- terminus of the thylakoid- targeting sequence and a pH gradient across the inner membrane are required for transport of the folded protein into the thylakoid lumen (step 3). The translocon in the thylakoid membrane is composed of at least four proteins related to proteins in the bacterial plasma membrane. The thylakoid- targeting sequence containing the two arginine residues is cleaved in the thylakoid lumen (step 4). See R. Dalbey and C. Robinson, 1999, Trends Biochem. Sci. 24:17; R. E. Dalbey and A. Kuhn, 2000, Annu. Rev. Cell Dev. Biol. 16:51; and C. Robinson and A. Bolhuis, 2001, Nat. Rev. Mol. Cell Biol. 2:350.
[Leave] [Close]