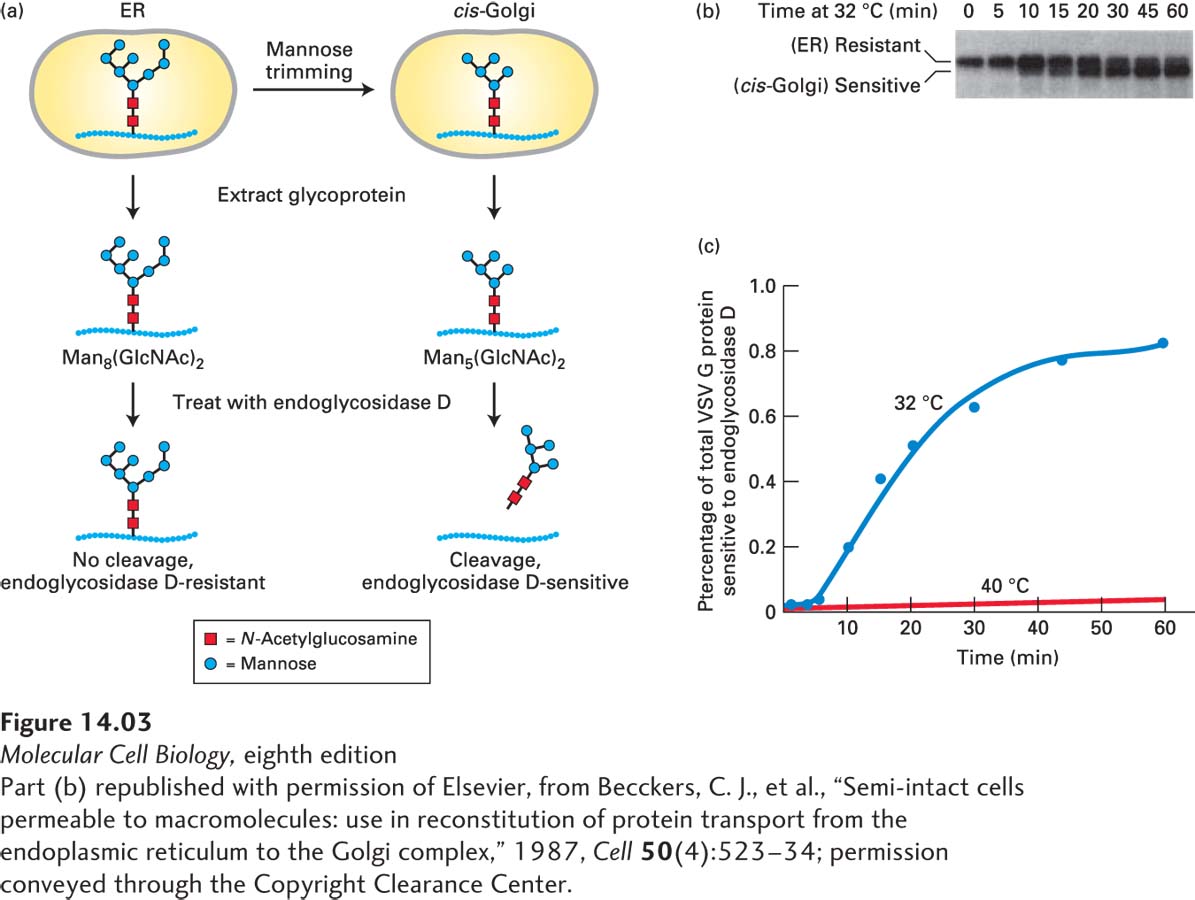
EXPERIMENTAL FIGURE 14- 3 Transport of a membrane glycoprotein from the ER to the Golgi can be assayed based on sensitivity to cleavage by endoglycosidase D. Cells expressing a temperature- sensitive VSV G protein were labeled with a pulse of radioactive amino acids at the nonpermissive temperature so that the labeled protein was retained in the ER. At periodic times after a return to the permissive temperature of 32 °C, VSV G protein was extracted from cells and digested with endoglycosidase D. (a) As proteins move to the cis-Golgi from the ER, the core oligosaccharide Man8(GlcNAc)2 is trimmed to Man5(GlcNAc)2 by enzymes that reside in the cis-Golgi compartment. Endoglycosidase D cleaves the oligosaccharide chains from proteins processed in the cis-Golgi, but not from proteins in the ER. (b) SDS- polyacrylamide gel electrophoresis of the digestion mixtures resolves the resistant, uncleaved (slower- migrating) and sensitive, cleaved (faster- migrating) forms of labeled VSV G protein. Initially, as this gel shows, all of the VSV G protein was resistant to digestion, but over time, an increasing fraction was sensitive to digestion, reflecting transport of the protein from the ER to the Golgi and its processing there. In control cells kept at 40 °C, only slow- moving, digestion- resistant VSV G protein was detected after 60 minutes (not shown). (c) A plot of the percentage of VSV G protein that is sensitive to digestion, derived from electrophoretic data, reveals the time course of ER- to- Golgi transport.
[Part (b) republished with permission of Elsevier, from Becckers, C. J., et al., “Semi- intact cells permeable to macromolecules: use in reconstitution of protein transport from the endoplasmic reticulum to the Golgi complex,” 1987, Cell 50(4):523– 34; permission conveyed through the Copyright Clearance Center.]
[Leave] [Close]