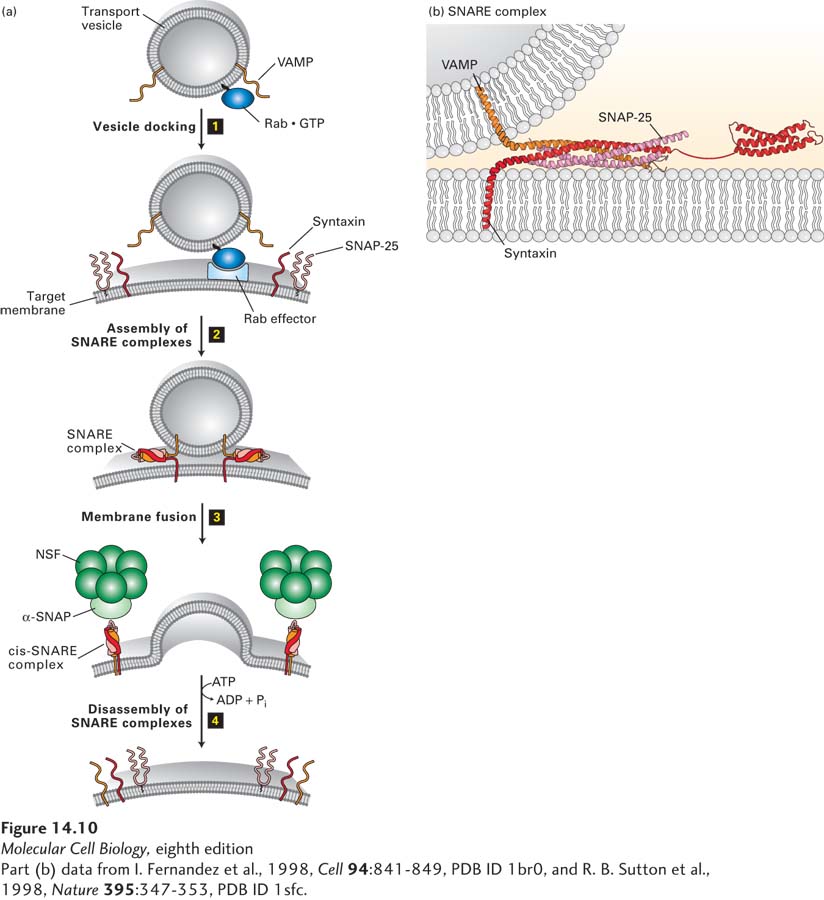
FIGURE 14- 10 Model for docking and fusion of transport vesicles with their target membranes. (a) The proteins shown in this example participate in fusion of secretory vesicles with the plasma membrane, but similar proteins mediate all vesicle- fusion events. Step 1: A Rab protein tethered via a lipid anchor to a secretory vesicle binds to an effector protein complex on the plasma membrane, thereby docking the transport vesicle on the appropriate target membrane. Step 2: A v- SNARE protein (in this case, VAMP) interacts with the cytosolic domains of the cognate t- SNAREs (in this case, syntaxin and SNAP- 25). The very stable coiled- coil SNARE complexes that are formed hold the vesicle close to the target membrane. Step 3: Fusion of the two membranes immediately follows formation of SNARE complexes, but precisely how this occurs is not known. Step 4: Following membrane fusion, NSF, in conjunction with α-SNAP, binds to the SNARE complexes. The NSF- catalyzed hydrolysis of ATP then drives dissociation of the SNARE complexes, freeing the SNARE proteins for another round of vesicle fusion. Also at this time, Rab·GTP is hydrolyzed to Rab·GDP and dissociates from the Rab effector (not shown). (b) The SNARE complex. Numerous noncovalent interactions between four long α helices, two from SNAP- 25 and one each from syntaxin and VAMP, stabilize the coiled- coil structure. See J. E. Rothman and T. Söllner, 1997, Science 276:1212, Y. A. Chen and R. H. Scheller, 2001, Nat. Rev. Mol. Cell Biol. 2:98, and W. Weis and R. Scheller, 1998, Nature 395:328.
[Part (b) data from I. Fernandez et al., 1998, Cell 94:841- 849, PDB ID 1br0, and R. B. Sutton et al., 1998, Nature 395:347- 353, PDB ID 1sfc.]
[Leave] [Close]