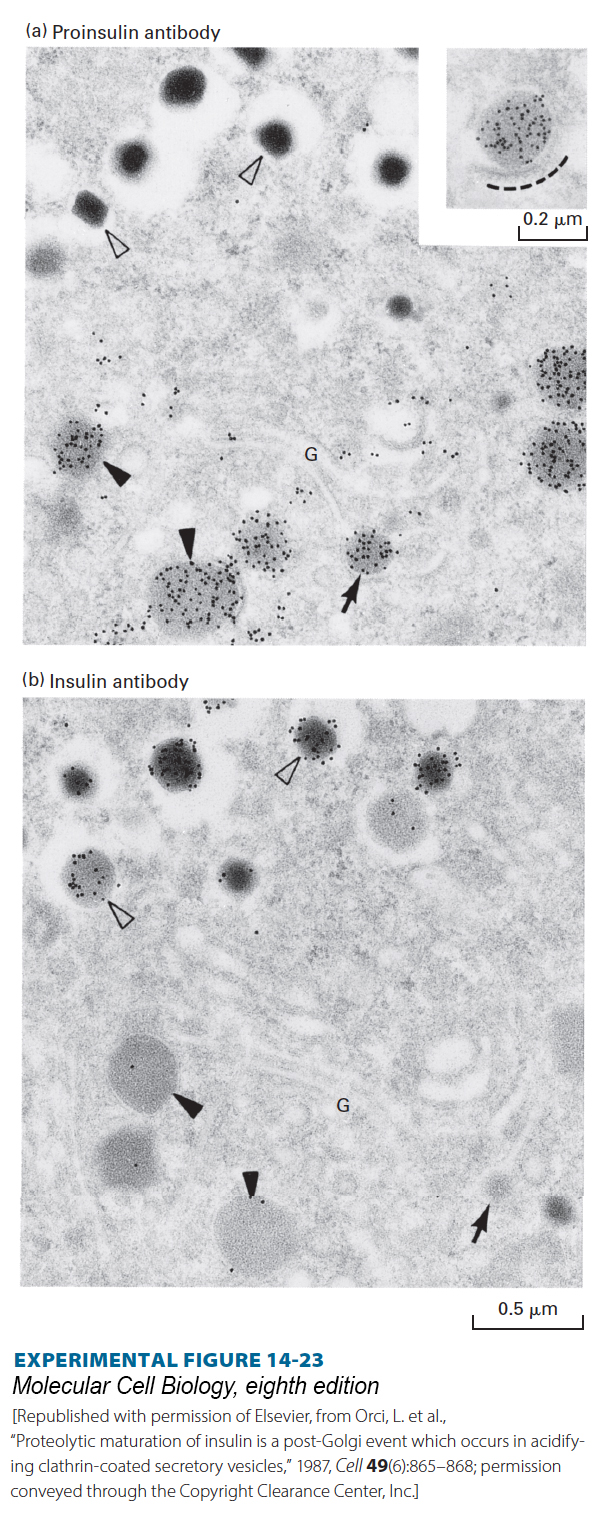
EXPERIMENTAL FIGURE 14- 23 Proteolytic cleavage of proinsulin occurs in secretory vesicles after they have budded from the trans-Golgi network. Serial sections of the Golgi region of an insulin- secreting cell were stained with (a) a monoclonal antibody that recognizes proinsulin, but not insulin, or (b) a different antibody that recognizes insulin, but not proinsulin. The antibodies, which were bound to electron- opaque gold particles, appear as dark dots in these electron micrographs (see Figure 4- 33 ). Immature secretory vesicles (closed arrowheads) and vesicles budding from the trans-Golgi (arrows) stain with the proinsulin antibody, but not with the insulin antibody. These vesicles contain diffuse protein aggregates that include proinsulin and other regulated secreted proteins. Mature vesicles (open arrowheads) stain with insulin antibody, but not with proinsulin antibody, and have a dense core of almost crystalline insulin. Since budding and immature secretory vesicles contain proinsulin (not insulin), the proteolytic conversion of proinsulin to insulin must take place in these vesicles after they bud from the trans-Golgi network. The inset in (a) shows a proinsulin- rich secretory vesicle surrounded by a protein coat (dashed line).
[Republished with permission of Elsevier, from Orci, L. et al., “Proteolytic maturation of insulin is a post- Golgi event which occurs in acidifying clathrin- coated secretory vesicles,” 1987, Cell 49(6):865– 868; permission conveyed through the Copyright Clearance Center, Inc.]
[Leave] [Close]