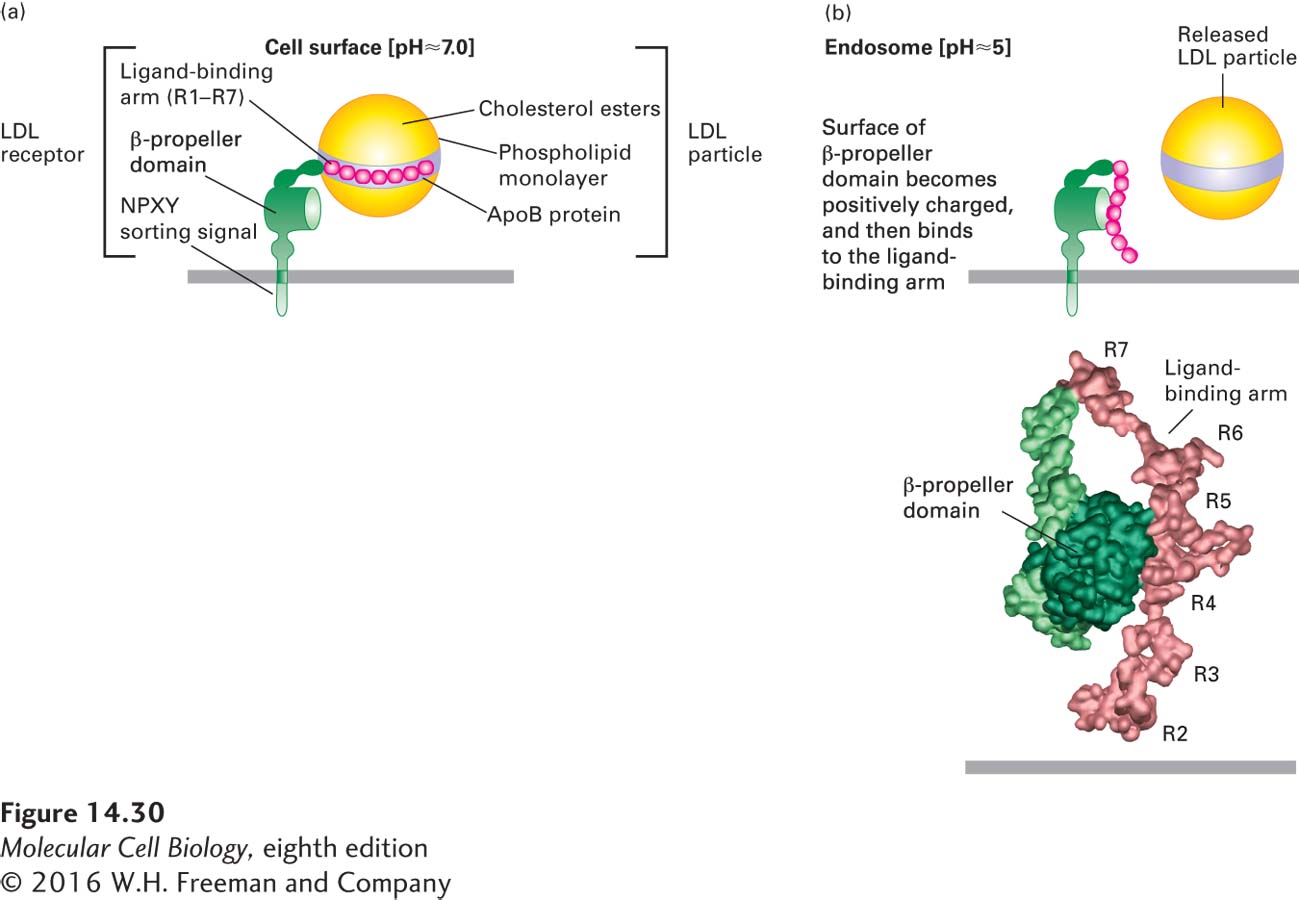
FIGURE 14- 30 Model for pH- dependent binding of LDL particles by the LDL receptor. Schematic depiction of an LDL receptor at the neutral pH found at the cell surface (a) and at the acidic pH found in the interior of the late endosome (b). (a) At the cell surface, apoB- 100 on the surface of an LDL particle binds tightly to the receptor. Of the seven cysteine- rich repeats (R1– R7) in the ligand- binding arm, R4 and R5 appear to be most critical for LDL binding. (b, top) Within the endosome, histidine residues in the β-propeller domain of the LDL receptor become protonated. The positively charged propeller can bind with high affinity to the ligand- binding arm, which contains negatively charged residues, causing release of the LDL particle. (b, bottom) Experimental electron density and Cα backbone trace model of the extracellular region of the LDL receptor at pH 5.3 based on x- ray crystallographic analysis. In this conformation, extensive hydrophobic and ionic interactions occur between the β propeller and the R4 and R5 repeats.
[Part (b) data from G. Rudenko et al., 2002, Science 298:2353, PDB ID 1n7d.]
[Leave] [Close]