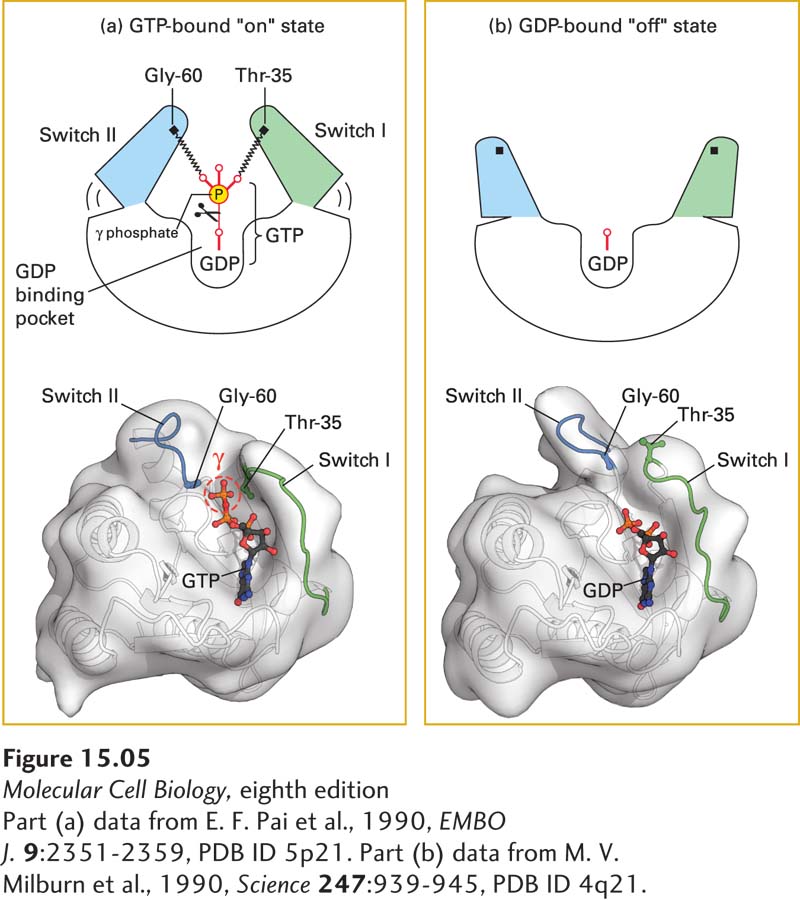
FIGURE 15- 5 Switching mechanism of monomeric G proteins. The ability of a G protein to interact with other proteins and thus transduce a signal differs between the GTP- bound “on” state and GDP- bound “off” state. (a) In the active “on” state, two domains, termed switch I (green) and switch II (blue), are bound to the terminal γ phosphate of GTP through interactions with the backbone amide groups of conserved threonine and glycine residues. When bound to GTP in this way, the two switch domains are in a conformation such that they can bind to and thus activate specific downstream effector proteins. (b) Removal of the γ phosphate by GTPase- catalyzed hydrolysis causes switch I and switch II to relax into a different conformation, the inactive “off” state; in this state, they are unable to bind to effector proteins. The three- dimensional models shown here represent both conformations of Ras, a monomeric G protein. A similar spring- loaded mechanism switches the alpha subunit in heterotrimeric G proteins between the active and inactive conformations by movement of three, rather than two, switch segments.
[Part (a) data from E. F. Pai et al., 1990, EMBO J. 9:2351- 2359, PDB ID 5p21. Part (b) data from M. V. Milburn et al., 1990, Science 247:939- 945, PDB ID 4q21.]
[Leave] [Close]