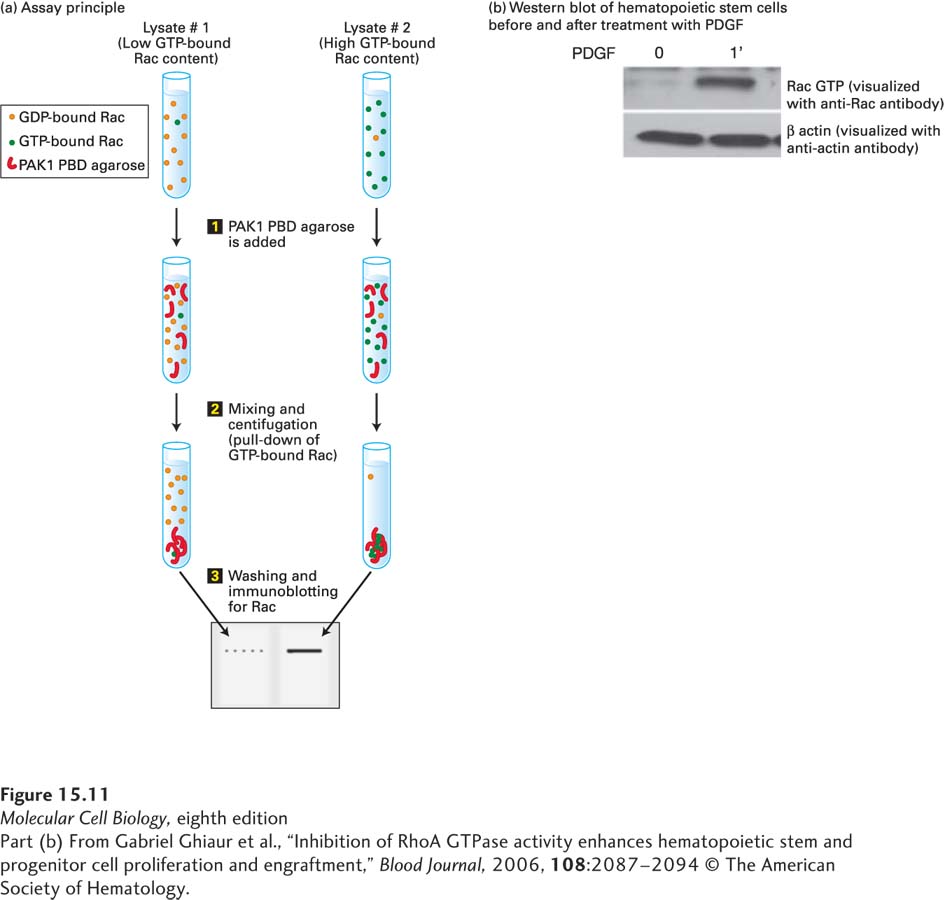
EXPERIMENTAL FIGURE 15- 11 A pull- down assay shows that the small GTP- binding protein Rac is activated by platelet- derived growth factor (PDGF). Like other small GTPases, Rac regulates molecular events by cycling between an inactive GDP- bound form and an active GTP- bound form. In its active (GTP- bound) state, Rac binds specifically to the Rac binding (PBD) domain of p21- activated protein kinase (PAK1) to control downstream signaling cascades. (a) Assay principle: The Rac- binding PBD domain is generated by recombinant DNA techniques and attached to agarose beads, then mixed with cell extracts (step 1). The beads are specifically recovered by centrifugation (step 2), and the amount of GTP- bound Rac is quantified by Western blotting using an anti- Rac antibody (step 3). (b) Western blot showing activation of Rac after treatment of hematopoietic stem cells for 1 minute with the hormone platelet- derived growth factor (PDGF). A Western blot for actin serves as a control to show that the same amount of total protein is loaded on each lane of the gel.
[Part (b) From Gabriel Ghiaur et al., “Inhibition of RhoA GTPase activity enhances hematopoietic stem and progenitor cell proliferation and engraftment,” Blood Journal, 2006, 108:2087– 2094 © The American Society of Hematology.]
[Leave] [Close]