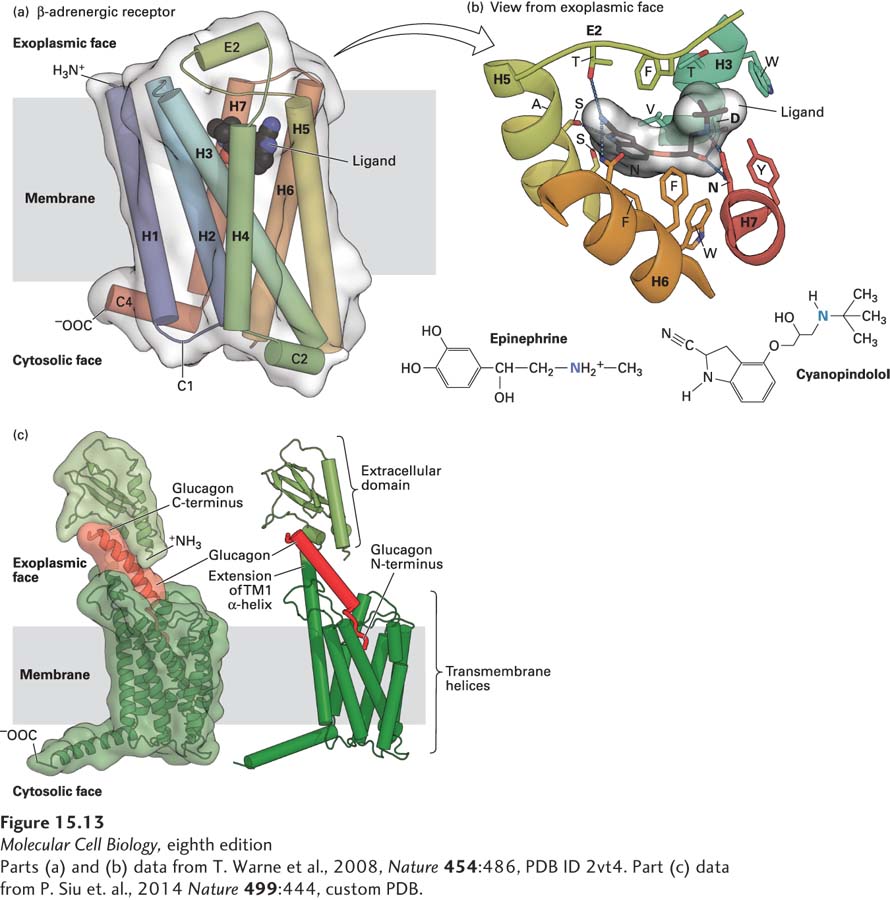
FIGURE 15- 13 Binding of ligands to GPCRs. (a) Structure of the turkey β1-adrenergic receptor bound to its antagonist cyanopindolol. This side view shows the approximate location of the membrane phospholipid bilayer. The ribbon representation of the receptor structure is in rainbow coloration (N- terminus, blue; C- terminus, red), with cyanopindolol as a gray space- filling molecule. The extracellular loop 2 (E2) and cytoplasmic loops 1, 2, and 4 (C1, C2, C4) 1 and 2 (C1, C2) are labeled. (b) The hormone- binding pocket formed by residues in several transmembrane segments. View from external face showing a close- up of the ligand- binding pocket that is formed by amino acids in helices 3, 5, 6, and 7, as well as extracellular loop 2, located between helices 4 and 5. Cyanopindolol atoms are colored gray (carbon), blue (nitrogen), and red (oxygen). The ligand- binding pocket comprises 15 side chains from amino acid residues in four transmembrane α helices and extracellular loop 2. As examples of specific binding interactions, the positively charged N atom in the amino group found both in cyanopindolol and in epinephrine forms an ionic bond with the carboxylate side chain of aspartate 121 (D) in helix 3 and the carboxylate of asparagine 329 (N) in helix 7. (c) Model for glucagon binding to the glucagon receptor. The seven transmembrane α helices of the glucagon receptor and the exoplasmic extension of transmembrane helix 1 are colored dark green and the N- terminal exoplasmic domain light green. The C- terminus of the 29- amino- acid peptide glucagon (red) is bound to the receptor N- terminal domain, and the glucagon N- terminus is thought to insert into a binding pocket that is in the center of the seven transmembrane α helices.
[Parts (a) and (b) data from T. Warne et al., 2008, Nature 454:486, PDB ID 2vt4. Part (c) data from P. Siu et al., 2014 Nature 499:444, custom PDB.]
[Leave] [Close]