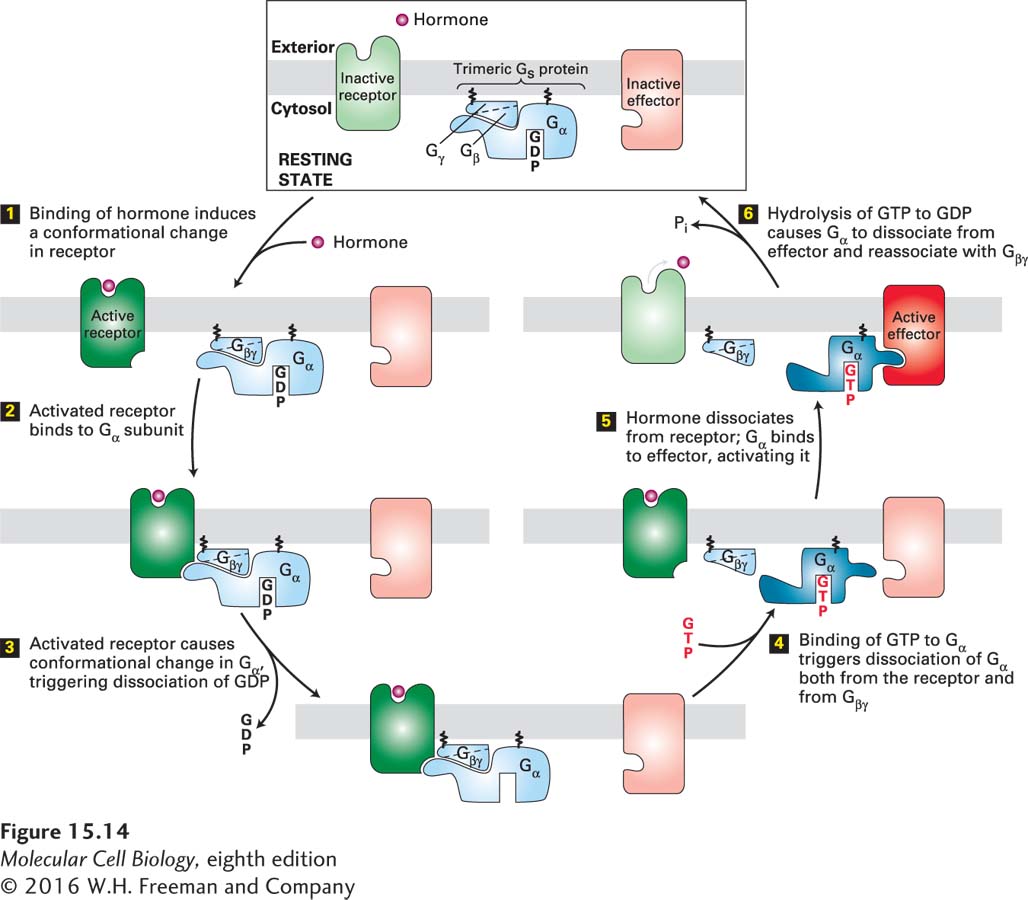
FIGURE 15- 14 General mechanism of the activation of effector proteins associated with G protein– coupled receptors. Light colors denote the inactive and dark colors the active conformations of each protein. The Gα and Gβγ subunits of a heterotrimeric G protein are tethered to the membrane by covalently attached lipid molecules (wiggly black lines). Following ligand binding, exchange of GDP for GTP, and dissociation of the G protein subunits (steps 1 – 4), the free Gα·GTP binds to and activates an effector protein (step 5). Hydrolysis of GTP terminates signaling and leads to reassembly of the heterotrimeric G protein, returning the system to the resting state (step 6). Binding of another ligand molecule causes repetition of the cycle. In some pathways, the effector protein is activated by the free Gβγ subunit. See W. Oldham and H. Hamm, 2006, Quart. Rev. Biophys. 39:117.
[Leave] [Close]