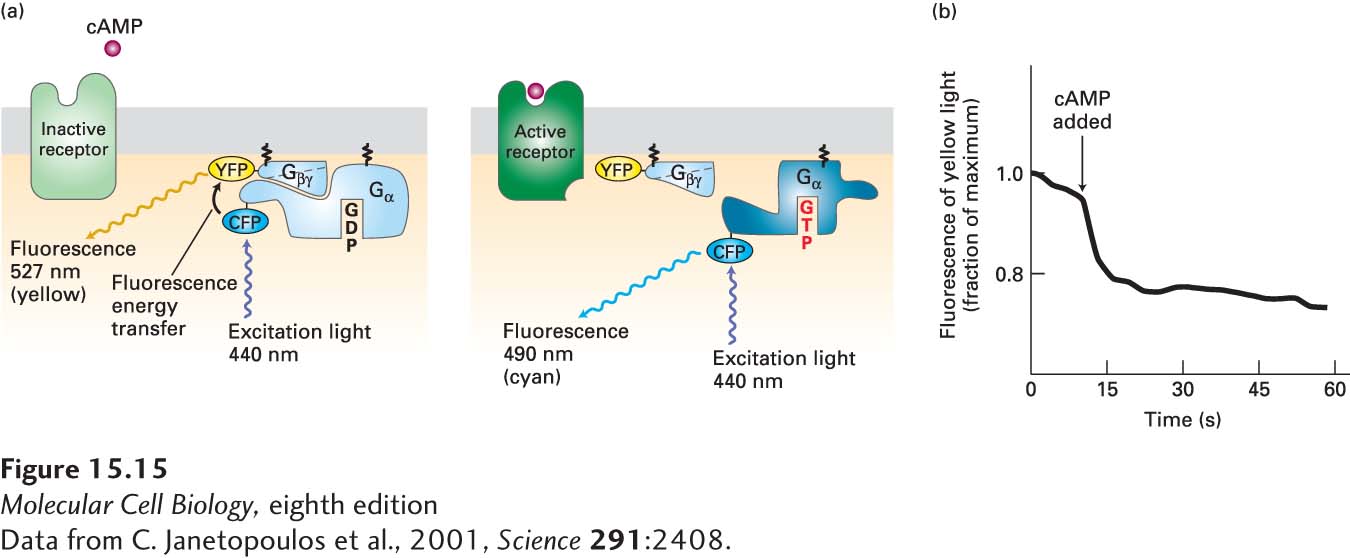
EXPERIMENTAL FIGURE 15- 15 Activation of a G protein occurs within seconds of ligand binding to its cell- surface G protein– coupled receptor. In the amoeba Dictyostelium discoideum, cAMP acts as an extracellular signaling molecule and binds to and signals via a G protein– coupled receptor; it is not a second messenger. Amoeba cells were transfected with genes encoding two fusion proteins: a Gα fused to cyan fluorescent protein (CFP), and a Gβ fused to yellow fluorescent protein (YFP). CFP normally fluoresces 490- nm light; YFP, 527- nm light. (a) When CFP and YFP are close to each other, as in the resting Gα·Gβγ complex, fluorescence energy transfer can occur between them (left). As a result, irradiation of resting cells with 440- nm light (which directly excites CFP but not YFP) causes emission of 527- nm (yellow) light, characteristic of YFP, because of fluorescence energy transfer from CFP to YFP. However, if ligand binding leads to dissociation of the Gα and Gβγ subunits, then fluorescence energy transfer cannot occur. In this case, irradiation of cells at 440 nm causes emission of 490- nm (cyan) light, characteristic of CFP (right). (b) Plot of the emission of yellow light (527 nm) from a single transfected amoeba cell before and after addition of extracellular cAMP (arrow). The drop in yellow fluorescence, which results from the dissociation of the Gα-CFP fusion protein from the Gβ-YFP fusion protein, occurs within seconds of cAMP addition.
[Data from C. Janetopoulos et al., 2001, Science 291:2408.]
[Leave] [Close]