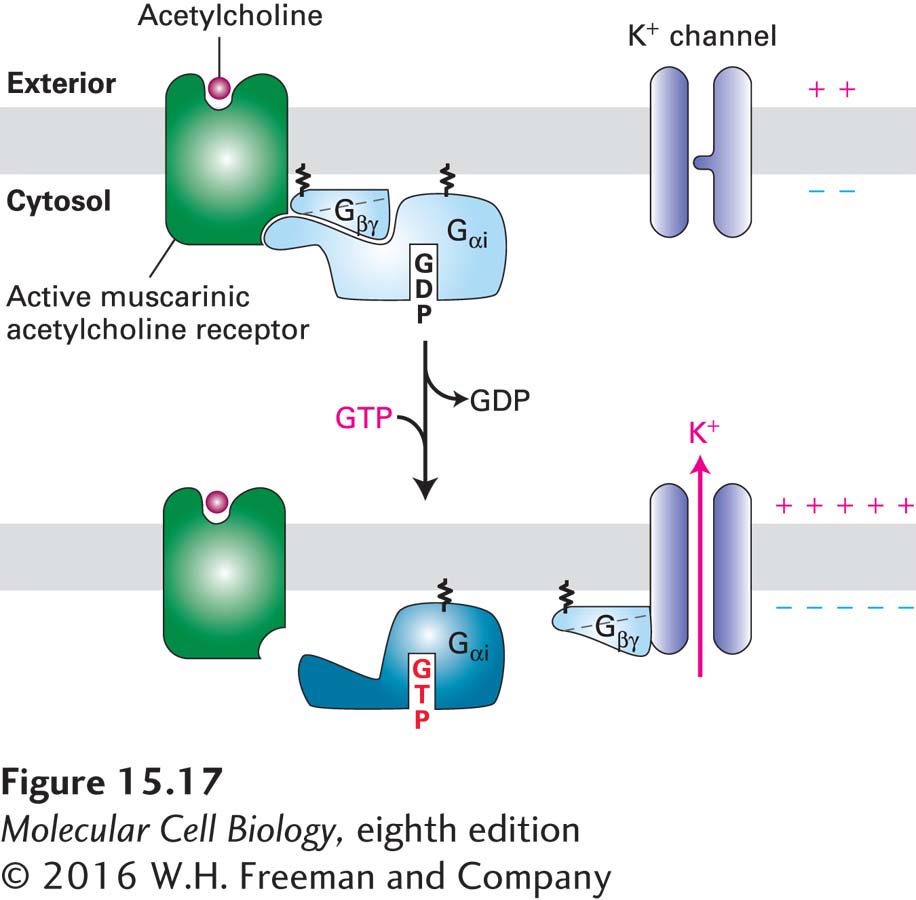
FIGURE 15- 17 In heart muscle, the muscarinic acetylcholine receptor activates its effector K+ channel via the Gβγ subunit of a Gi protein. Binding of acetylcholine triggers activation of the Gαi subunit and its dissociation from the Gβγ subunit in the usual way (see Figure 15- 14 ). In this case, however, the released Gβγ subunit (rather than Gαi·GTP) binds to and opens the associated effector protein, a K+ channel. The increase in K+ permeability hyperpolarizes the membrane, which reduces the frequency of heart muscle contraction. Though not shown here, activation is terminated when the GTP bound to Gαi is hydrolyzed (by a GAP enzyme that is an intrinsic part of the Gαi subunit) to GDP and Gαi·GDP recombines with Gβγ. See K. Ho et al., 1993, Nature 362:31, and Y. Kubo et al., 1993, Nature 362:127.
[Leave] [Close]