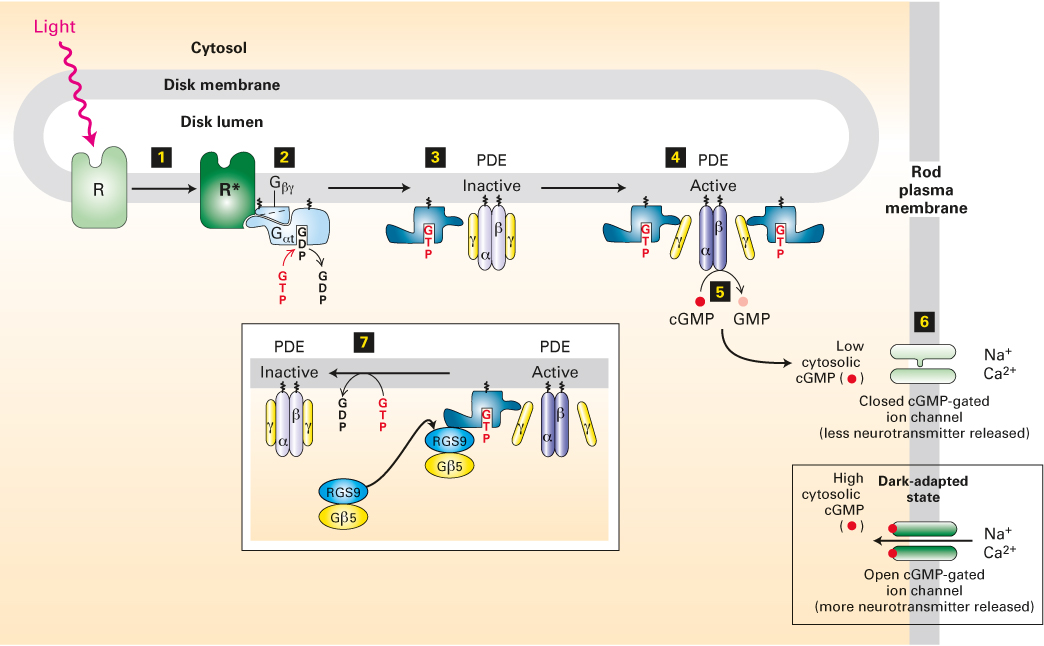
FIGURE 15- 20 The light- activated rhodopsin pathway and the closing of cation channels in rod cells. In dark- adapted rod cells, a high level of cGMP keeps cGMP- gated nonselective cation channels open, leading to depolarization of the plasma membrane and neurotransmitter release. Light absorption generates activated rhodopsin, R* (step 1), which binds inactive, GDP- bound Gαt protein and mediates the exchange of GDP for GTP (step 2). The free Gαt·GTP generated then activates PDE by binding to its inhibitory γ subunits (step 3) and dissociating them from the catalytic α and β subunits (step 4). Relieved of their inhibition, the α and β subunits of PDE hydrolyze cGMP to GMP (step 5). The resulting decrease in cytosolic cGMP leads to dissociation of cGMP from the cation channels in the plasma membrane and the closing of those channels (step 6). The membrane then becomes transiently hyperpolarized, and neurotransmitter release is reduced. The complex of Gαt·GTP and the PDE γ subunits binds a GTPase- activating complex termed RGS9- Gβ5 (step 7); by hydrolyzing the bound GTP, this complex triggers the physiologically important rapid inactivation of the PDE. See V. Arshavsky and E. Pugh, 1998, Neuron 20:11, and V. Arshavsky, 2002, Trends Neurosci. 25:124.
[Leave] [Close]