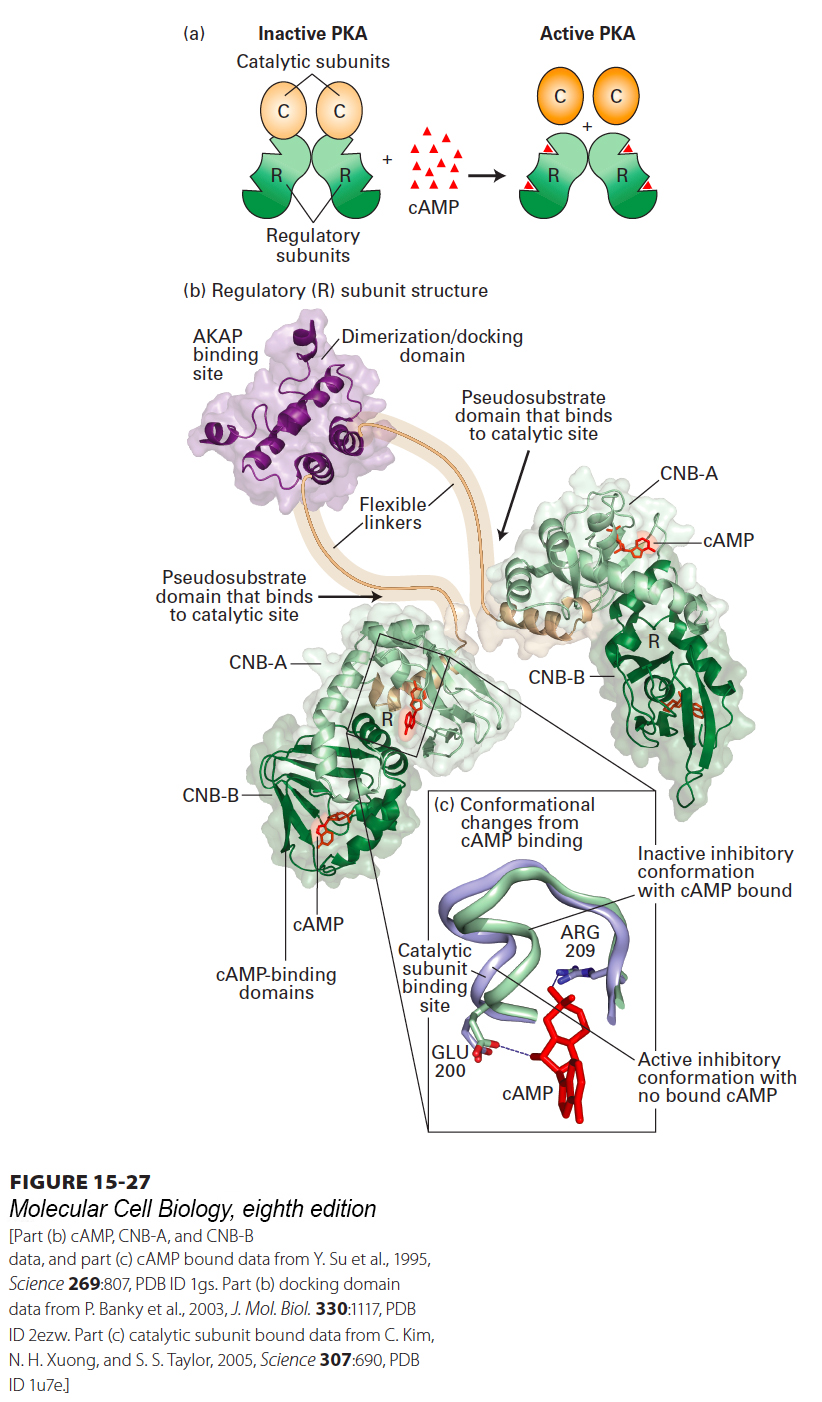
FIGURE 15- 27 Structure of PKA and its activation by cAMP. (a) PKA consists of two regulatory (R) subunits and two catalytic (C) subunits. When cAMP (red triangle) binds to the regulatory subunit, the catalytic subunit is released, thus activating PKA. (b) The two regulatory subunits form a dimer, joined by a dimerization/docking domain and a flexible linker to which an A kinase– associated protein (AKAP; see Figure 15- 31 ) can bind. Each R subunit has two cAMP- binding domains, CNB- A and CNB- B, and a binding site for a catalytic subunit (arrow). (c) Binding of cAMP to the CNB- A domain causes a subtle conformational change that displaces the C subunit from the R subunit, leading to its activation. Without bound cAMP, one loop of the CNB- A domain (purple) is in a conformation that can bind the catalytic (C) subunit. A glutamate (E200) and arginine (R209) residue participate in binding of cAMP (red), which causes a conformational change (green) in the loop that prevents binding of the loop to the C subunit.
[Part (b) cAMP, CNB- A, and CNB- B data, and part (c) cAMP bound data from Y. Su et al., 1995, Science 269:807, PDB ID 1gs. Part (b) docking domain data from P. Banky et al., 2003, J. Mol. Biol. 330:1117, PDB ID 2ezw. Part (c) catalytic subunit bound data from C. Kim, N. H. Xuong, and S. S. Taylor, 2005, Science 307:690, PDB ID 1u7e.]
[Leave] [Close]