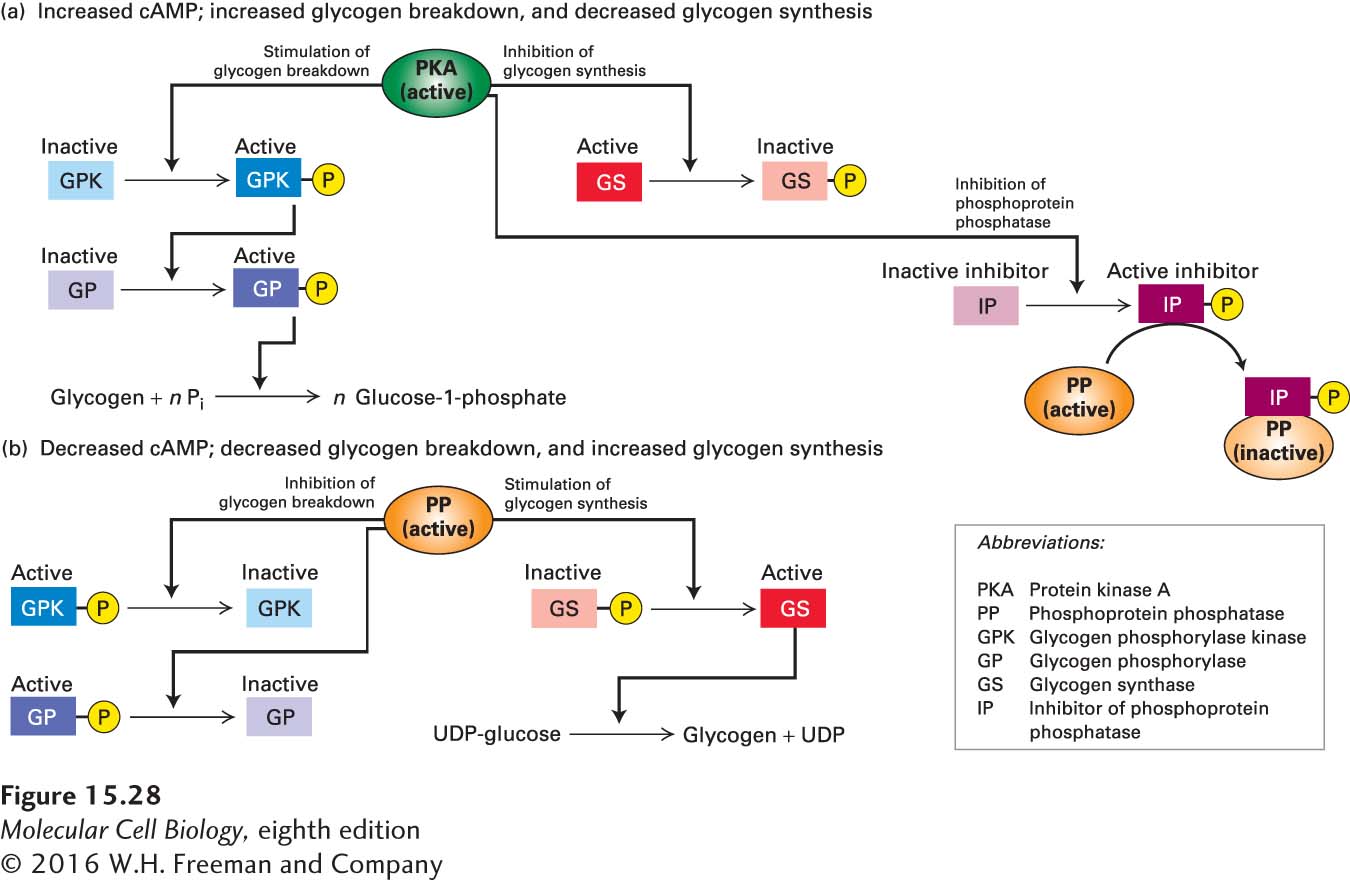
FIGURE 15- 28 Regulation of glycogen metabolism by cAMP and PKA. Active enzymes are highlighted in darker shades; inactive forms, in lighter shades. (a) An increase in cytosolic cAMP activates PKA, which phosphorylates glycogen synthase (GS) and thus inhibits glycogen synthesis directly. Active PKA also promotes glycogen degradation via a protein kinase cascade. At high cAMP concentrations, PKA also phosphorylates an inhibitor of phosphoprotein phosphatase (PP). Binding of the phosphorylated inhibitor to PP prevents this phosphatase from de- phosphorylating the activated enzymes in the kinase cascade or the inactive glycogen synthase. (b) A decrease in cAMP inactivates PKA, leading to release of the active form of PP. The activation of PP promotes glycogen synthesis and inhibits glycogen degradation.
[Leave] [Close]