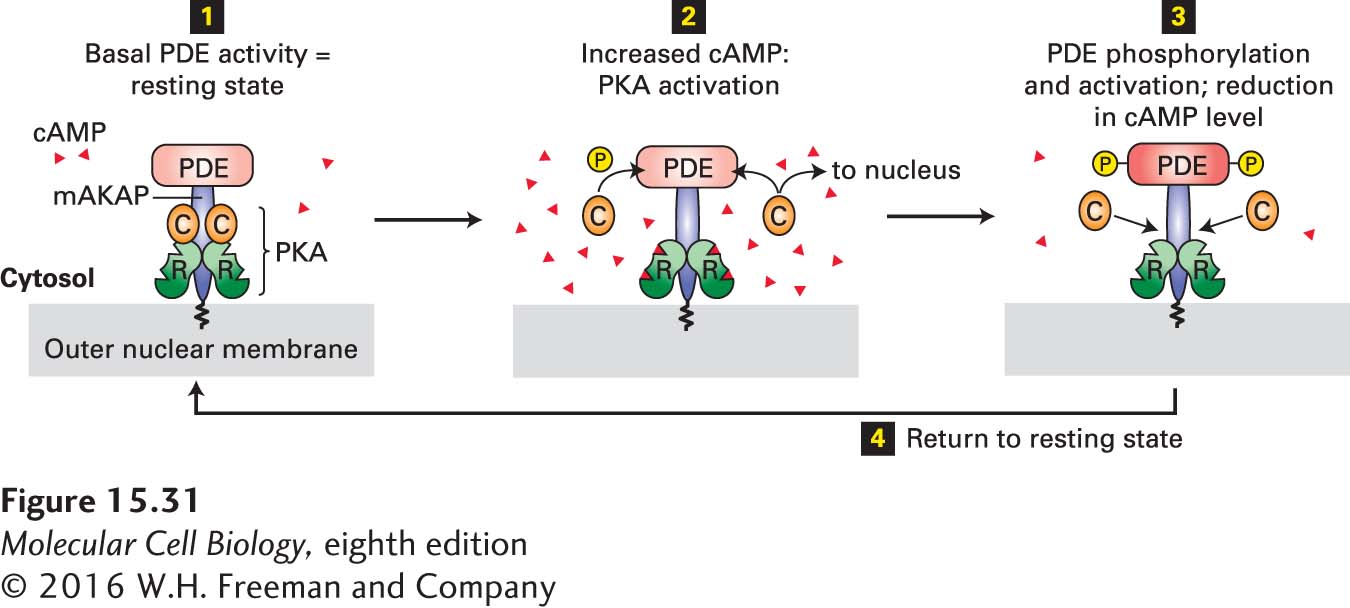
FIGURE 15- 31 Localization of PKA and PDE to the nuclear membrane in heart muscle by an A kinase– associated protein (AKAP). This member of the AKAP family, designated mAKAP, anchors both PDE and the regulatory subunit (R, see Figure 15- 27b ) of PKA to the nuclear membrane, maintaining them in a negative feedback loop that provides close local control of the cAMP level and PKA activity. Step 1: The basal level of PDE activity in the absence of hormone (resting state) keeps cAMP levels below those necessary for PKA activation. Steps 2 and 3: Activation of β-adrenergic receptors causes an increase in cAMP to a level in excess of that which can be degraded by PDE. The resulting binding of cAMP to the R subunits of PKA releases the active catalytic (C) subunits into the cytosol. Some C subunits enter the nucleus, where they phosphorylate and thus activate certain transcription factors (see Figure 15- 30 ). Other C subunits phosphorylate PDE, stimulating its catalytic activity. Active PDE hydrolyzes cAMP, thereby driving cAMP levels back to basal levels and causing re- formation of the inactive PKA C- R complex. Step 4: Subsequent de- phosphorylation of PDE returns the complex to the resting state. See K. L. Dodge et al., 2001, EMBO J. 20:1921.
[Leave] [Close]