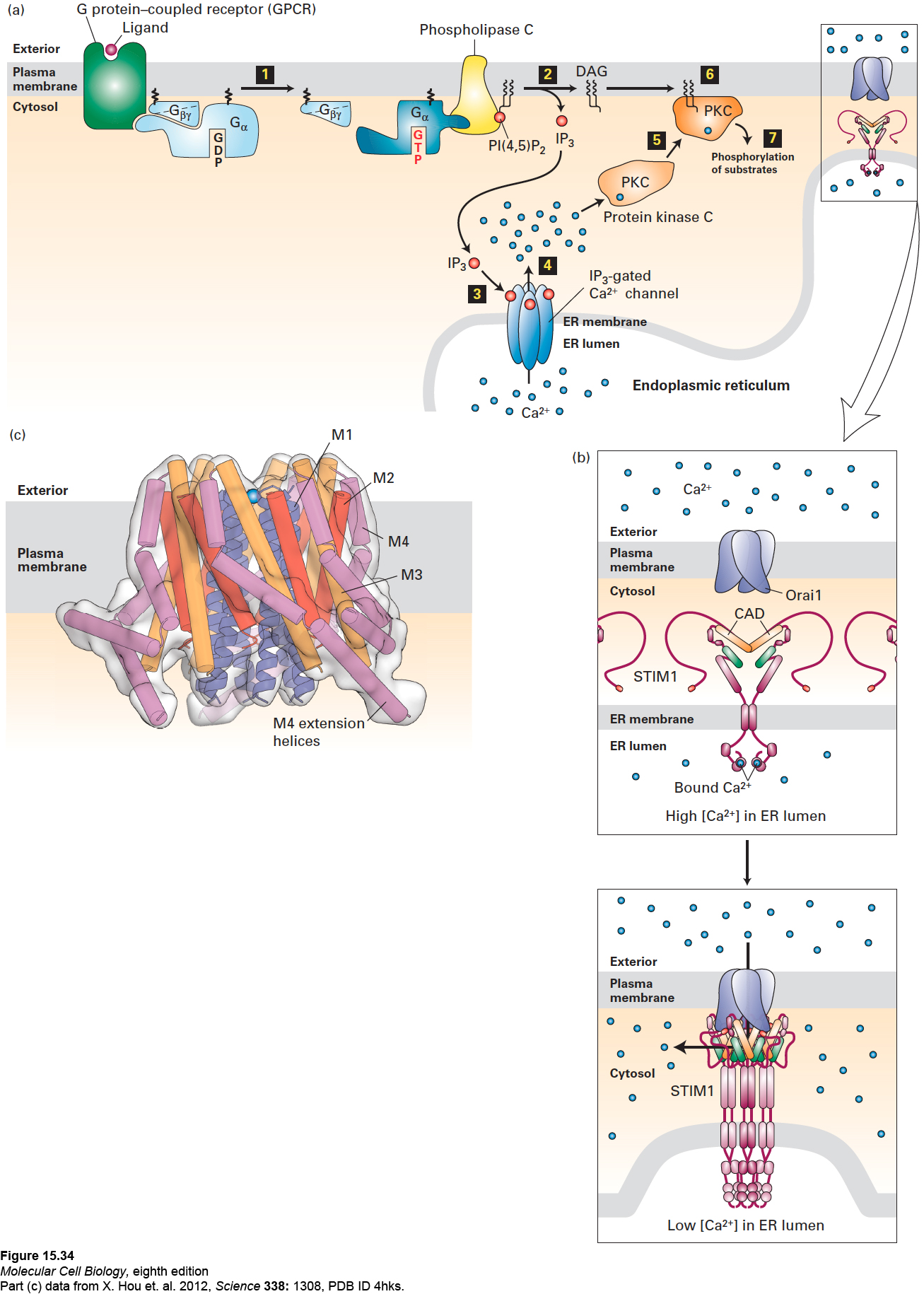
FIGURE 15- 34 The IP3/DAG pathway and the elevation of cytosolic Ca2+. (a) Opening of endoplasmic reticulum Ca2+ channels can be triggered by ligand binding to GPCRs that activate either the Gαo or Gαq subunit, leading to activation of phospholipase C (step 1). Cleavage of PI(4,5)P2 by phospholipase C yields IP3 and DAG (step 2). After diffusing through the cytosol, IP3 interacts with and opens IP3-gated Ca2+ channels in the membrane of the endoplasmic reticulum (step 3), causing release of stored Ca2+ ions into the cytosol (step 4). One of several cellular responses induced by a rise in cytosolic Ca2+ is recruitment of protein kinase C (PKC) to the plasma membrane (step 5), where it is activated by DAG (step 6). The activated membrane- associated kinase can phosphorylate various cellular enzymes and receptors, thereby altering their activity (step 7). (b) Opening of plasma- membrane Ca2+ channels. Top: In the resting cell, Ca2+ levels in the ER lumen are high, and Ca2+ ions (blue circles) bind to the luminal EF hand domains of the transmembrane STIM proteins. Bottom: As Ca2+ stores in the ER are depleted and Ca2+ ions dissociate from the EF hands, STIMs undergo oligomerization and relocalization to areas of the ER membrane near the plasma membrane. There the STIM CAD domains (orange) bind to and trigger the opening of the store- operated Ca2+ channels (Orai1) in the plasma membrane, allowing influx of extracellular Ca2+. (c) Drawing of the three- dimensional structure of Orai1 in the closed state. The Orai1 Ca2+ channel is composed of six identical subunits arranged around a central Ca2+ pore. Each subunit contains four transmembrane α helices— M1 (blue), M2 (red), M3 (orange), and M4 (violet)—and a helix following M4 that extends into the cytosol (termed the M4 extension helix, violet). The M1 helices are drawn as ribbons, the M2– M4 helices as cylinders. The pore is lined by the six M1 helices; in the closed state, a Ca2+ ion is bound at the extracellular entrance to the pore, but cannot enter it. Binding of the CADs leads to channel opening, most likely by widening of the pore by the outward movement of the M1 helices. The intracellular ends of the M1 helices are thought to interact with a portion of the STIM CAD, as are the M4 extensions, and it is hypothesized that the CADs bridge the cytosolic portions of the M1 helices and the M4/M4 extension helices. See J. W. Putney, 1999, P. Natl. Acad. Sci. USA 96:14669; Y. Zhou, 2010, P. Natl. Acad. Sci. USA 107:4896; and M. Cahalan, 2010, Science 330:43.
[Part (c) data from X. Hou et al. 2012, Science 338:1308, PDB ID 4hks.]
[Leave] [Close]