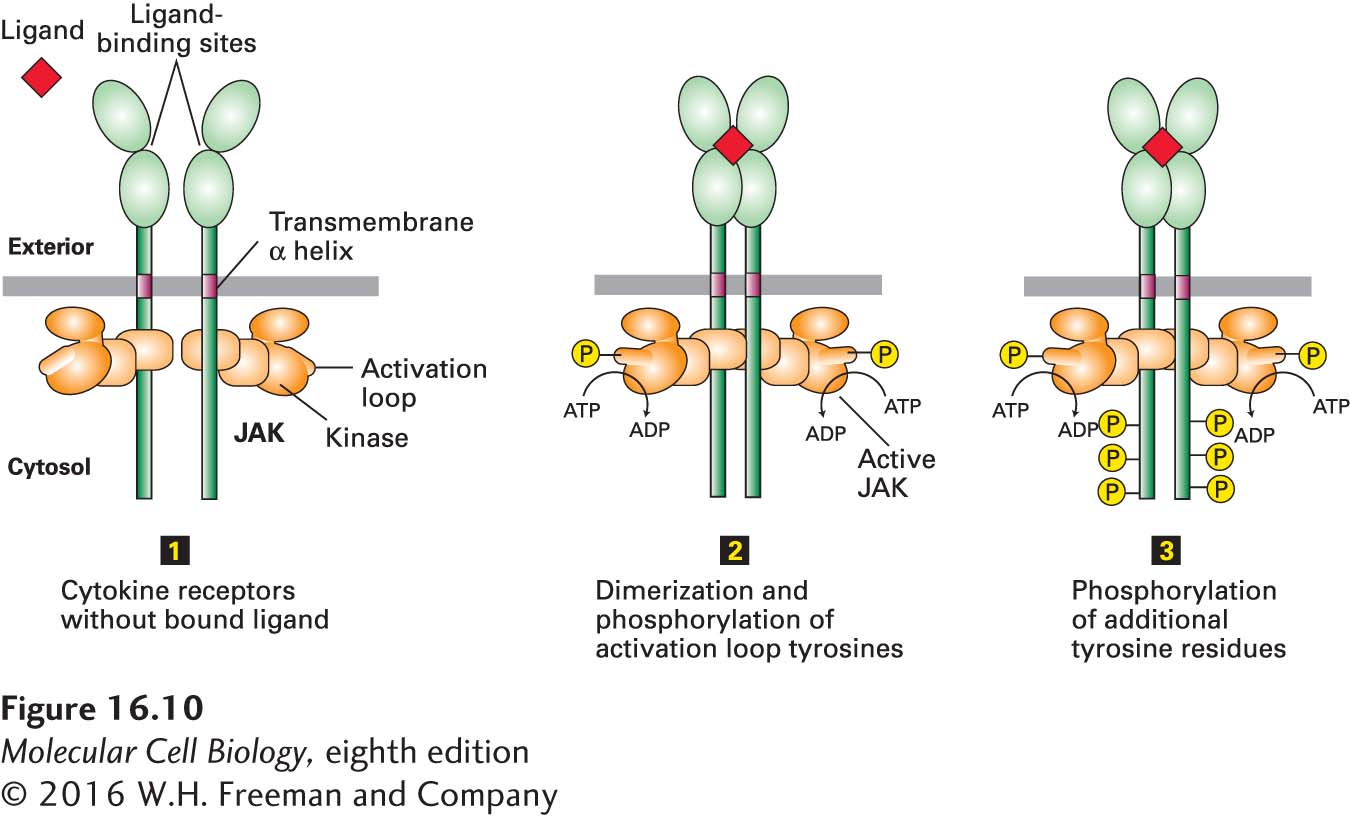
FIGURE 16- 10 General structure and activation of cytokine receptors. The cytosolic domain of a cytokine receptor binds tightly and irreversibly to a JAK protein tyrosine kinase. In the absence of ligand (step 1), two receptors form a homodimer, but the JAK kinases are poorly active. Ligand binding causes a conformational change that brings together the JAK kinase domains, which then phosphorylate each other on a tyrosine residue in a region called the activation loop, activating the kinases (step 2). The active JAK kinases then phosphorylate multiple tyrosine residues in the receptor cytosolic domain (step 3). The resulting phosphotyrosines function as docking sites for signal- transducing proteins, including the STAT proteins.
[Leave] [Close]