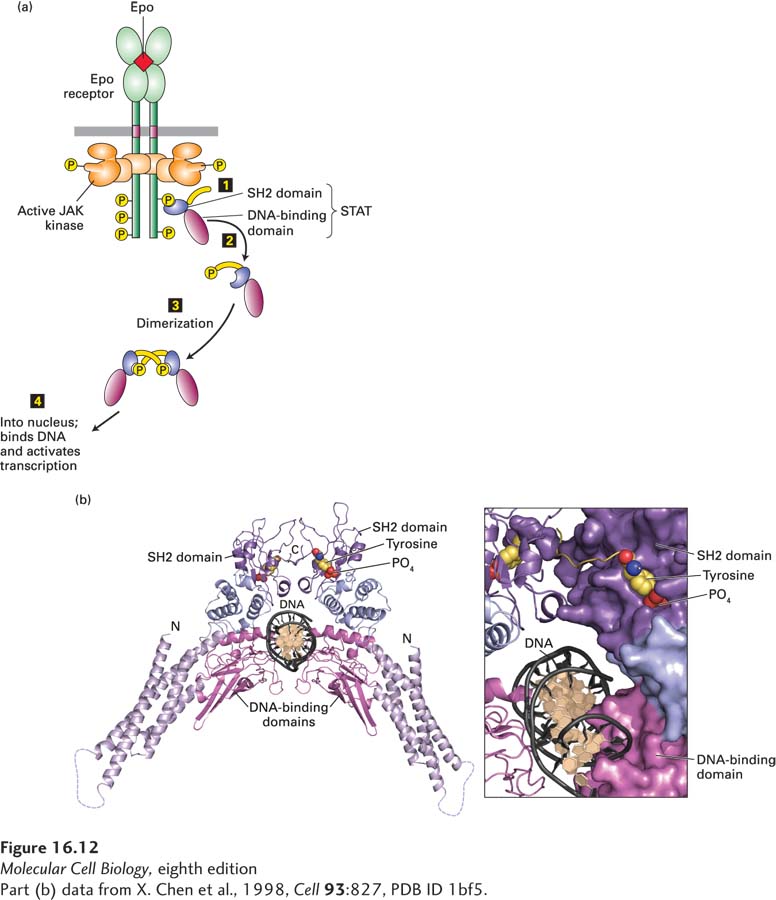
FIGURE 16- 12 Activation and structure of STAT proteins. (a) Phosphorylation and dimerization of STAT proteins. Step 1: Following activation of a cytokine receptor (see Figure 16- 10 ), the SH2 domain of an inactive monomeric STAT transcription factor binds to a phosphotyrosine in the receptor, bringing the STAT close to the active JAK associated with the receptor. The JAK then phosphorylates the C- terminal tyrosine in the STAT. Steps 2 and 3: Phosphorylated STATs spontaneously dissociate from the receptor and spontaneously dimerize. Because the STAT homodimer has two phosphotyrosine- SH2 domain interactions, whereas the receptor- STAT complex is stabilized by only one such interaction, phosphorylated STATs tend not to rebind to the receptor. Step 4: The STAT dimer moves into the nucleus, where it can bind to promoter sequences and activate transcription of target genes. (b) Ribbon diagram of the STAT1 dimer bound to DNA (black). The STAT1 dimer forms a C- shaped clamp around DNA that is stabilized by reciprocal and highly specific interactions between the SH2 domain (purple) of one monomer and the phosphorylated tyrosine residue (yellow with red oxygens) on the C- terminal segment of the other. The phosphotyrosine- binding site of the SH2 domain in each monomer is coupled structurally to the DNA- binding domain (magenta), suggesting a potential role for the SH2- phosphotyrosine interaction in the stabilization of DNA interacting elements.
[Part (b) data from X. Chen et al., 1998, Cell 93:827, PDB ID 1bf5.]
[Leave] [Close]