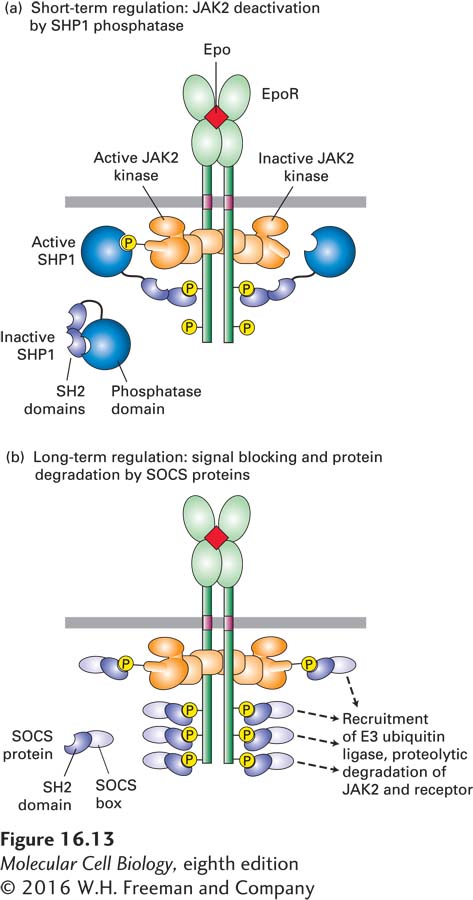
FIGURE 16- 13 Two mechanisms for terminating cytokine signal transduction as exemplified by the erythropoietin receptor (EpoR). (a) Short- term regulation: SHP1, a phosphotyrosine phosphatase, is present in an inactive form in the cytosol of unstimulated cells. Binding of an SH2 domain in SHP1 to a particular phosphotyrosine in the activated receptor unmasks its phosphatase catalytic site and positions it near the phosphorylated tyrosine in the activation loop region of JAK2. Removal of the phosphate from this tyrosine inactivates the JAK kinase. See S. Constantinescu et al., 1999, Trends Endocrin. Met. 10:18. (b) Long- term regulation: SOCS proteins, whose expression is induced by the STAT5 protein in erythropoietin- stimulated erythroid progenitor cells, inhibit or permanently terminate signaling over longer periods. Binding of SOCS to specific phosphotyrosine residues on EpoR or JAK2 blocks binding of other signaling proteins (left). The SOCS box also targets the receptor as well as JAK2 for degradation by the ubiquitin- proteasome pathway (right). Similar mechanisms regulate signaling from other cytokine receptors. See B. T. Kile and W. S. Alexander, 2001, Cell. Mol. Life Sci. 58:1627.
[Leave] [Close]