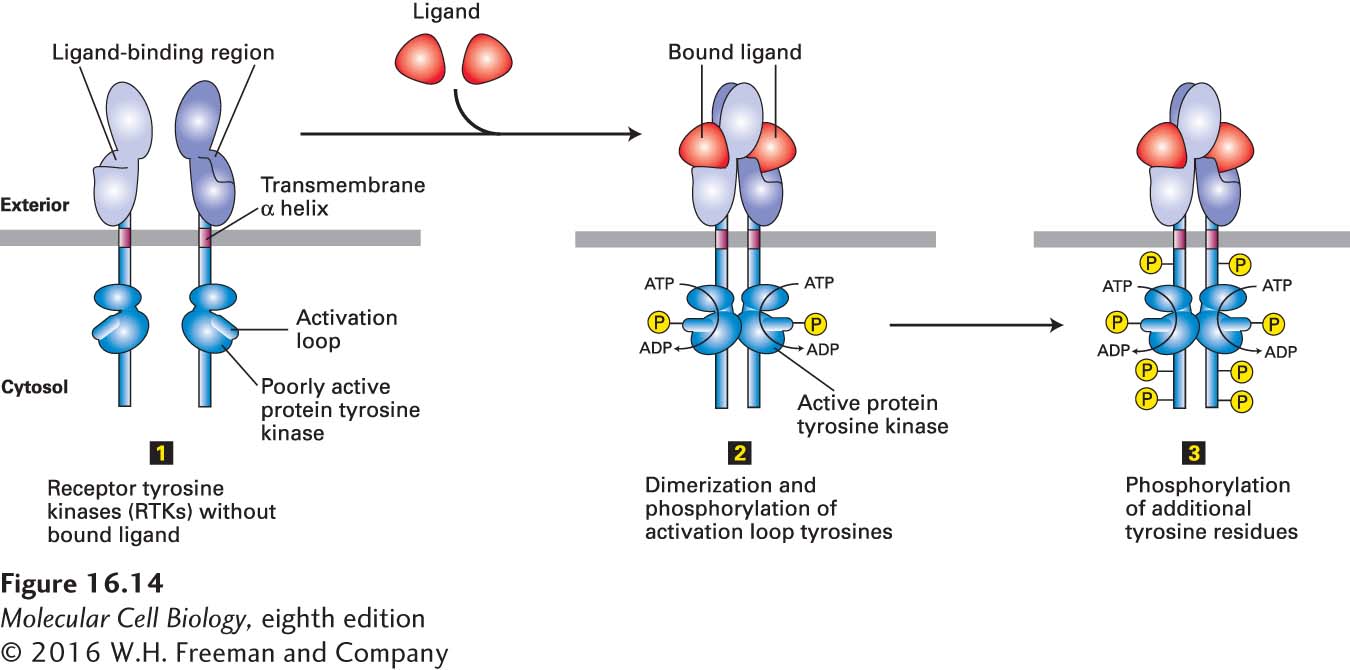
FIGURE 16- 14 General structure and activation of receptor tyrosine kinases (RTKs). The cytosolic domain of RTKs contains an intrinsic protein tyrosine kinase catalytic site. In the absence of ligand (step 1), RTKs generally exist as monomers with poorly active kinases. Binding of two ligands to the extracellular domains of two RTKs forms or stabilizes an activated dimeric receptor. This brings together two poorly active kinases such that each one phosphorylates the other on a tyrosine residue in the activation loop (step 2). Phosphorylation causes the loop to move out of the kinase catalytic site, thus increasing the ability of ATP and/or the protein substrate to bind. The activated kinase then phosphorylates several tyrosine residues in the receptor’s cytosolic domain (step 3). The resulting phosphotyrosines function as docking sites for SH2 and other binding domains on downstream signal- transducing proteins.
[Leave] [Close]