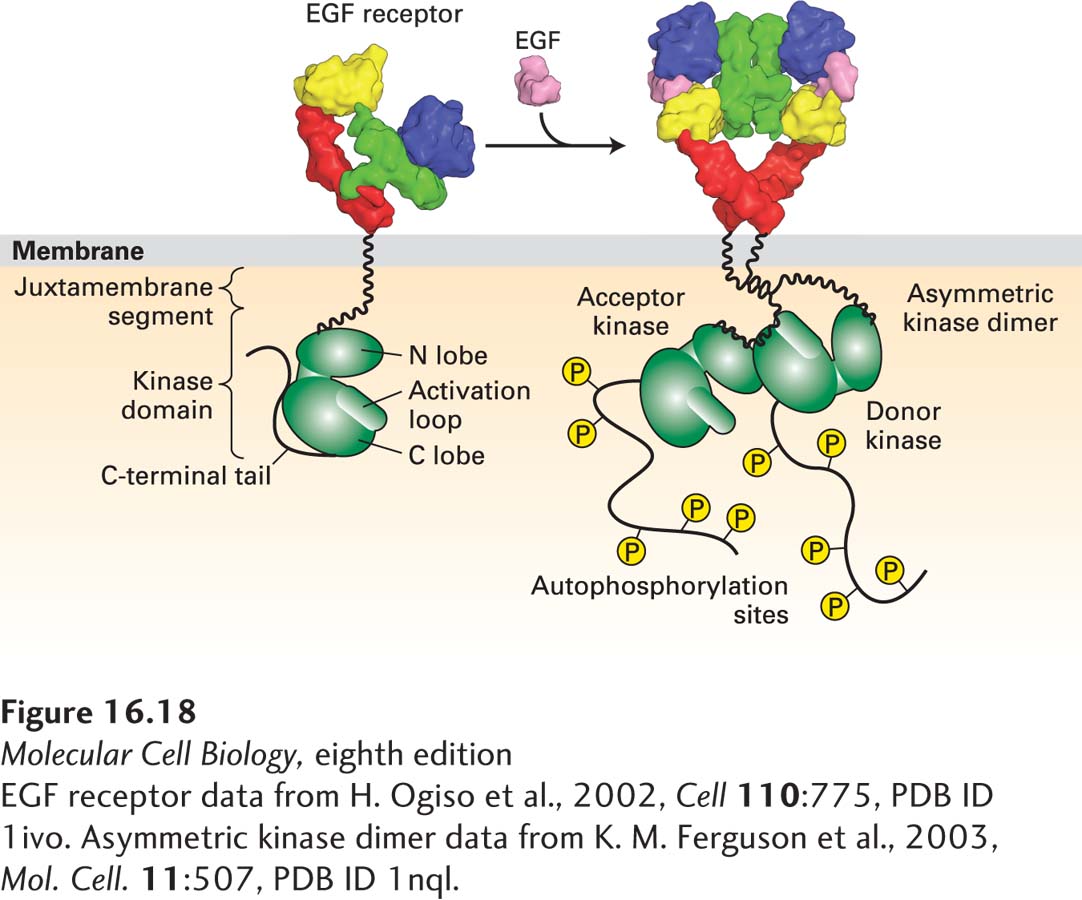
FIGURE 16- 18 Activation of the EGF receptor by EGF results in the formation of an asymmetric kinase domain dimer. In the inactive, monomeric state, the activation loop is localized to the kinase active site and thus inhibits kinase activation. Receptor dimerization generates an asymmetric kinase dimer such that the C- terminal C- lobe of the donor kinase binds to the N- terminal N- lobe of the acceptor kinase in the opposite receptor; the dimer is stabilized by interactions between the juxtamembrane segments of the two receptors. These interactions cause a conformational change that removes the activation loop from the kinase site of the acceptor kinase, activating its kinase activity. The active kinase then phosphorylates tyrosine residues in the C- terminal segments of the receptor cytosolic domain.
[EGF receptor data from H. Ogiso et al., 2002, Cell 110:775, PDB ID 1ivo. Asymmetric kinase dimer data from K. M. Ferguson et al., 2003, Mol. Cell. 11:507, PDB ID 1nql.]
[Leave] [Close]