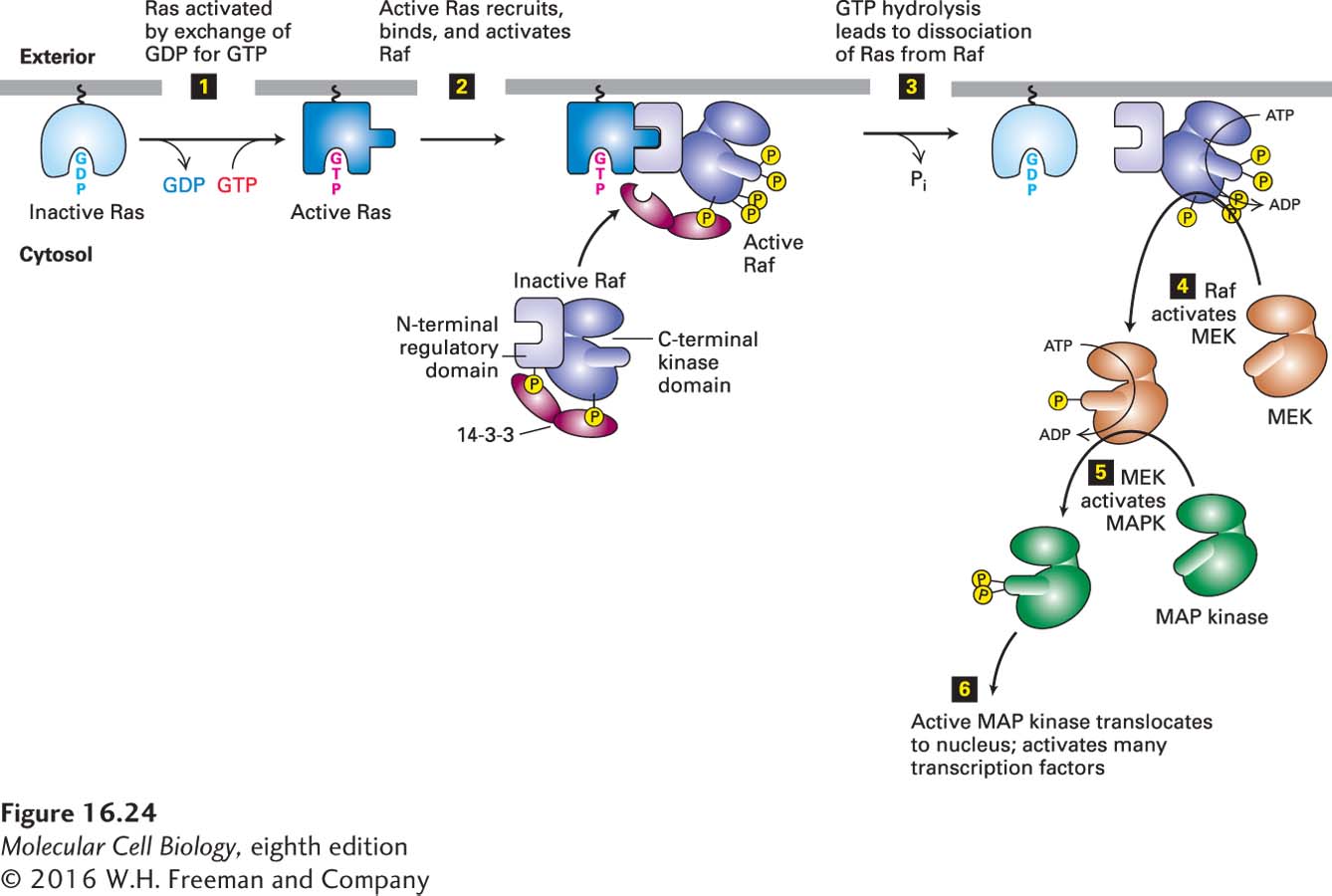
FIGURE 16- 24 Ras/MAP kinase pathway. In unstimulated cells, most Ras is tethered to the cytosolic surface of the plasma membrane in the inactive form with bound GDP. Binding of a ligand to its RTK or cytokine receptor leads to formation of the active Ras·GTP complex (step 1; see also Figure 16- 21 ). Activated Ras triggers the downstream kinase cascade depicted in steps 2–6, culminating in activation of MAP kinase (MAPK). In unstimulated cells, binding of a dimer of the 14- 3- 3 protein to Raf stabilizes it in an inactive conformation. Each 14- 3- 3 monomer binds to a phosphoserine residue in Raf, one to phosphoserine- 259 in the N- terminal domain and the other to phosphoserine- 621 in the kinase domain; binding to 14- 3- 3 maintains the kinase in a closed, inactive state. Interaction of the Raf N- terminal regulatory domain with Ras·GTP results in dephosphorylation of one of the serines that bind Raf to 14- 3- 3, phosphorylation of other residues, loss of 14- 3- 3 binding, and activation of Raf kinase activity. After inactive Ras·GDP dissociates from Raf, it can presumably be reactivated by signals from activated receptors, thereby recruiting additional Raf molecules to the membrane. See E. Kerkhoff and U. Rapp, 2001, Adv. Enzyme Regul. 41:261; J. Avruch et al., 2001, Recent Prog. Horm. Res. 56:127; and D. Matallanas et al., 2011, Genes Cancer 2:232.
[Leave] [Close]