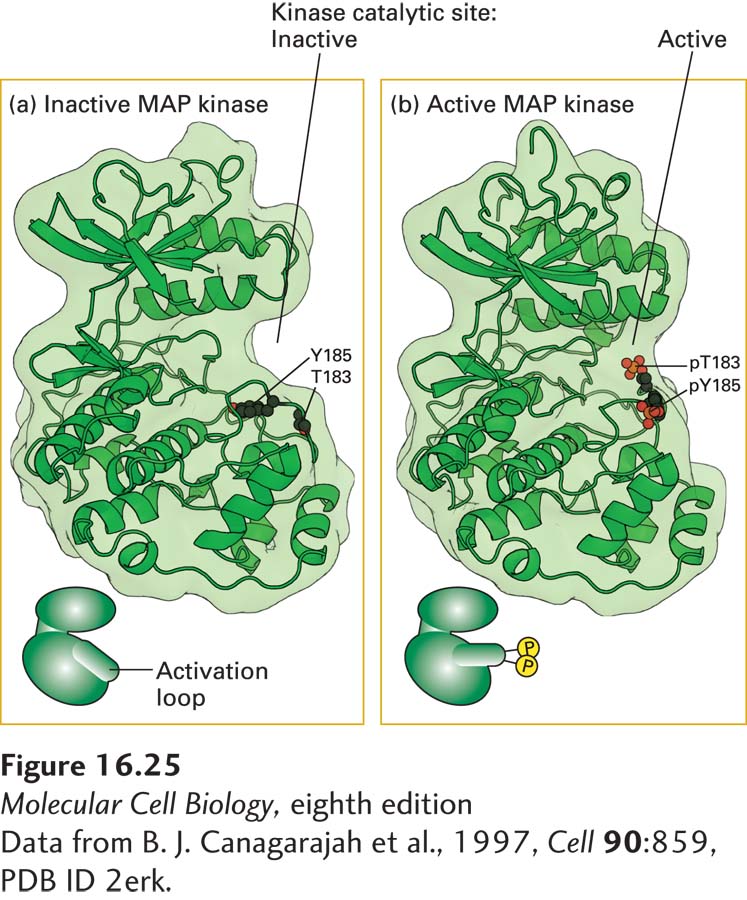
FIGURE 16- 25 Structures of inactive, nonphosphorylated MAP kinase and the active, phosphorylated form. (a) In inactive MAP kinase, the activation loop is in a conformation that blocks the kinase active site. (b) Phosphorylation by MEK at tyrosine 185 (Y- 185) and threonine 183 (T- 183) leads to a marked conformational change in the activation loop. This activating change promotes both binding of its substrates— ATP and its target proteins— to MAP kinase and its dimerization. A similar phosphorylation- dependent mechanism activates JAK kinases and the intrinsic kinase activity of RTKs.
[Data from B. J. Canagarajah et al., 1997, Cell 90:859, PDB ID 2erk.]
[Leave] [Close]