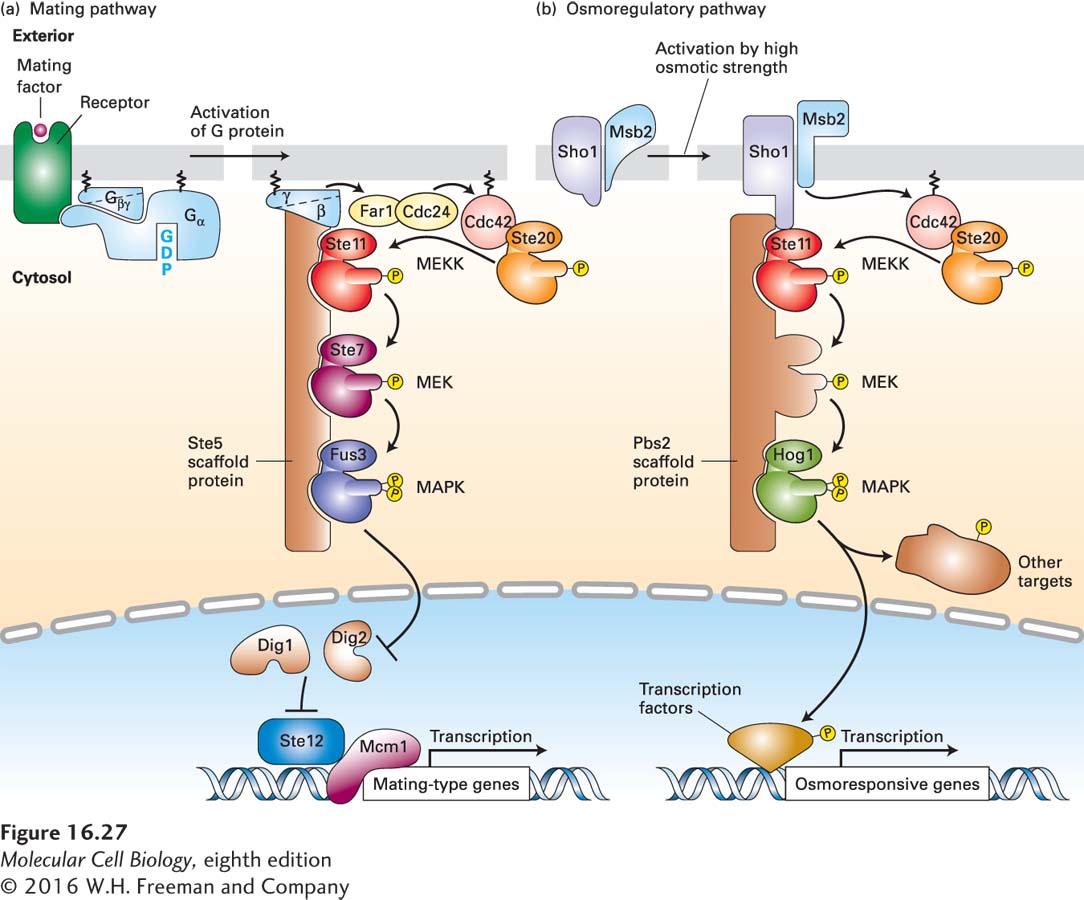
FIGURE 16- 27 Scaffold proteins separate yeast MAP kinase cascades in the mating and osmoregulatory pathways. In yeast, different receptors activate different MAP kinase pathways, two of which are outlined here. The two MEKs depicted, like all MEKs, are dual- specificity threonine/tyrosine kinases; all of the other kinases are serine/threonine kinases. (a) Mating pathway: The receptors for yeast α and a mating factors are coupled to the same trimeric G protein. Following ligand binding and dissociation of the G protein subunits, the membrane- tethered Gβγ subunit binds the Ste5 scaffold to the plasma membrane. Gβγ also activates Cdc24, a GEF for the Ras- like protein Cdc42; the active, GTP- bound Cdc42, in turn, binds to and activates the Ste20 kinase. Ste20 then phosphorylates and activates Ste11, which is analogous to Raf and other mammalian MEK kinase (MEKK) proteins. Ste20 thus serves as a MAPKKK kinase. Ste11 initiates a kinase cascade in which the final component, Fus3, is functionally equivalent to MAP kinase (MAPK) in higher eukaryotes. Like other MAP kinases, activated Fus3 then translocates into the nucleus. There it phosphorylates two proteins, Dig1 and Dig2, relieving their inhibition of the Ste12 transcription factor, allowing it to bind to DNA and initiate transcription of genes that inhibit progression of the cell cycle and others that enable cells of opposite mating type to fuse together and ultimately form a diploid cell. (b) Osmoregulatory pathway: Two plasma membrane proteins, Sho1 and Msb1, are activated in an unknown manner by exposure of yeast cells to media of high osmotic strength. Activated Sho1 recruits the Pbs2 scaffold protein, which contains a MEK domain, to the plasma membrane. At the plasma membrane, the Sho1- Msb1 complex activates Cdc42, which in turn activates the resident Ste20 kinase, as in the mating pathway. Ste20, in turn, phosphorylates and activates Ste11, initiating a kinase cascade that activates Hog1, a MAP kinase. In the cytosol, Hog1 phosphorylates specific protein targets, including ion channels; after translocating to the nucleus, Hog1 phosphorylates several transcription factors and chromatin- modifying enzymes. Hog1 also appears also to promote transcriptional elongation. Together, the newly synthesized and modified proteins support survival in high- osmotic- strength media. See N. Dard and M. Peter, 2006, BioEssays 28:146, and R. Chen and J. Thorner, 2007, Biochim. Biophys. Acta 1773:1311.
[Leave] [Close]