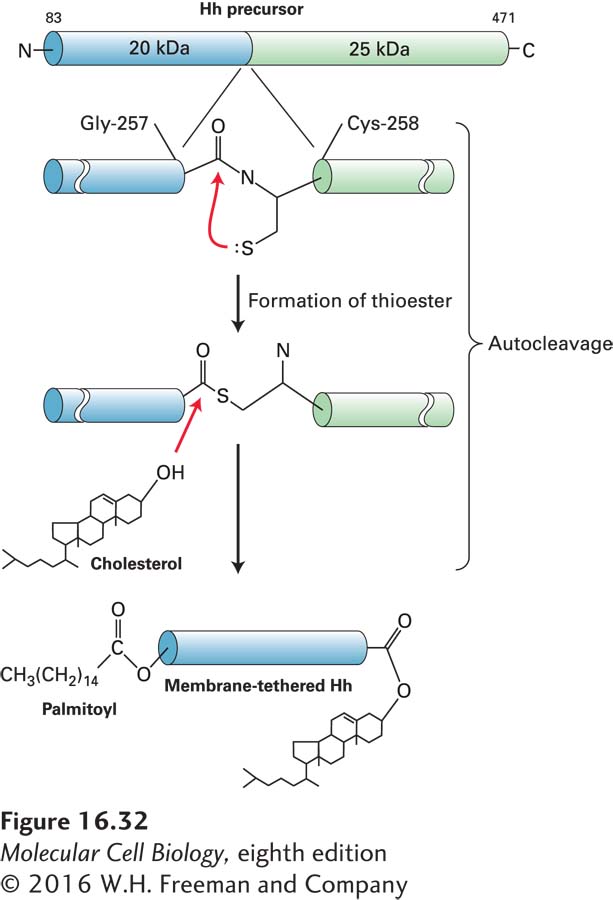
FIGURE 16- 32 Processing of Hedgehog (Hh) precursor protein. Cells synthesize a 45- kDa Hh precursor, which in the endoplasmic reticulum undergoes a nucleophilic attack by the thiol side chain of cysteine 258 (Cys- 258) on the carbonyl carbon of the adjacent residue glycine 257 (Gly- 257), forming a high- energy thioester intermediate. Enzyme activity in the C- terminal domain then catalyzes the formation of an ester bond between the hydroxyl group of cholesterol and glycine 257, cleaving the precursor into two fragments. The N- terminal signaling fragment (blue) retains the cholesterol group and is also modified by the addition of a palmitoyl group to the N- terminus. The two hydrophobic anchors may tether the secreted, processed Hh protein to the plasma membrane. See P. Thérond, 2012, Curr. Opin. Cell Biol. 24:173.
[Leave] [Close]