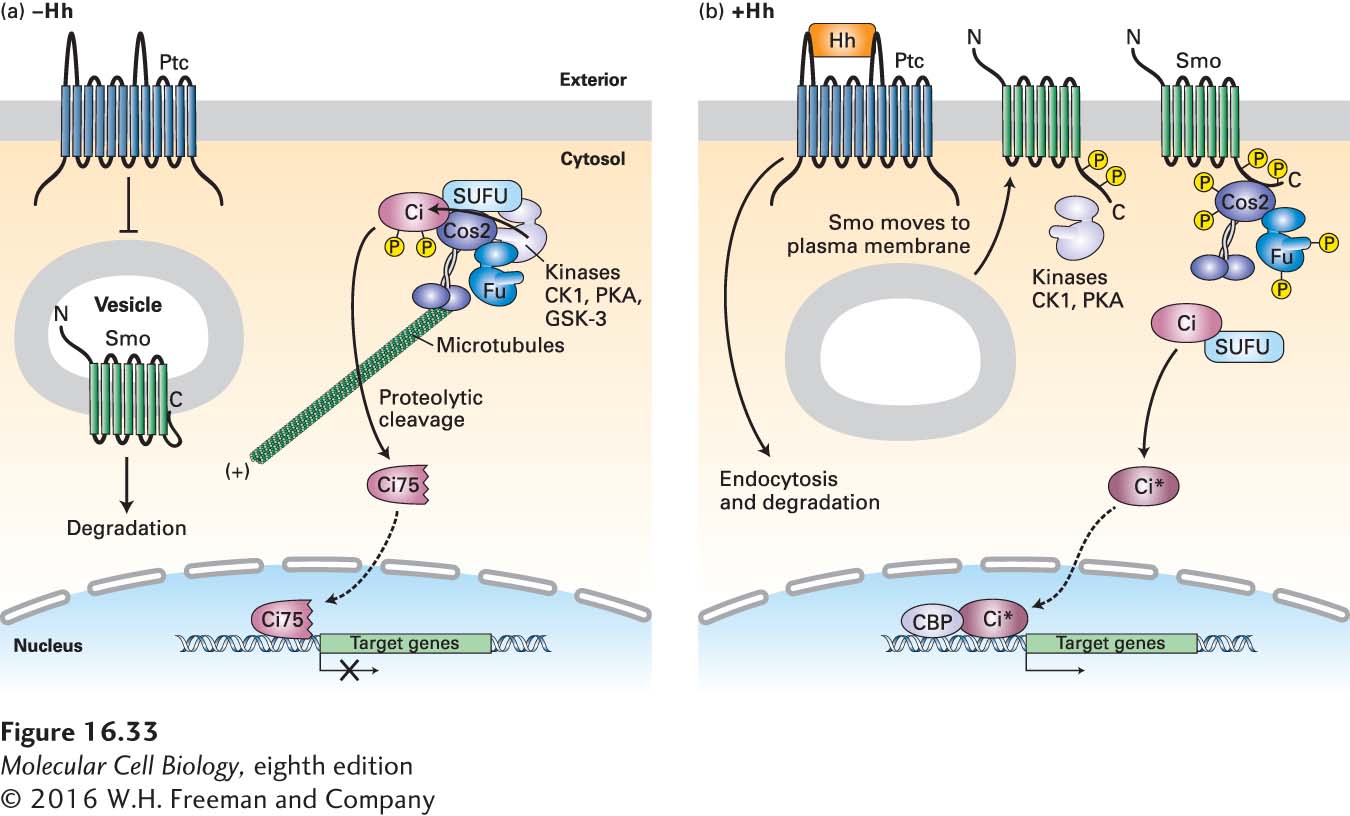
FIGURE 16- 33 Hedgehog signaling in Drosophila. (a) In the absence of Hedgehog (Hh), Patched (Ptc) protein inhibits Smoothened (Smo), which is present largely in the membranes of internal vesicles, and enhances its degradation. A complex containing the kinase Fused (Fu); other kinases including protein kinase A (PKA), glycogen synthase kinase 3β (GSK3β), and casein kinase 1 (CK1); the kinesin- related motor protein Costal- 2 (Cos2); and Cubitis interruptus (Ci), a zinc- finger transcription factor, binds to microtubules. In this complex, Ci becomes phosphorylated in a series of steps catalyzed by PKA, GSK3β, and CK1. The phosphorylated Ci is then proteolytically cleaved by the ubiquitin- proteasome pathway, generating the N- terminal fragment Ci75, which is transported into the nucleus and functions as a transcriptional repressor of Hh target genes. (b) Hh binds to Ptc, causing Ptc to be endocytosed from the cell surface and degraded, thereby relieving the inhibition of Smo. Smo then moves to the plasma membrane, is phosphorylated by PKA, CK1, and other kinases, binds Cos2, and is stabilized from degradation. Both Fu and Cos2 become extensively phosphorylated, and most importantly, the Fu- Cos2- Ci complex becomes dissociated. This leads to the stabilization of the full- length Ci, which moves into the nucleus, displaces the repressor Ci75 from the promoter of target genes, recruits the CREB- binding activator protein (CBP), and induces expression of target genes. The exact membrane compartments in which Ptc and Smo respond to Hh and function are unknown. See S. Goetz and K. Anderson, 2010, Nat. Rev. Genet. 11:331.
[Leave] [Close]