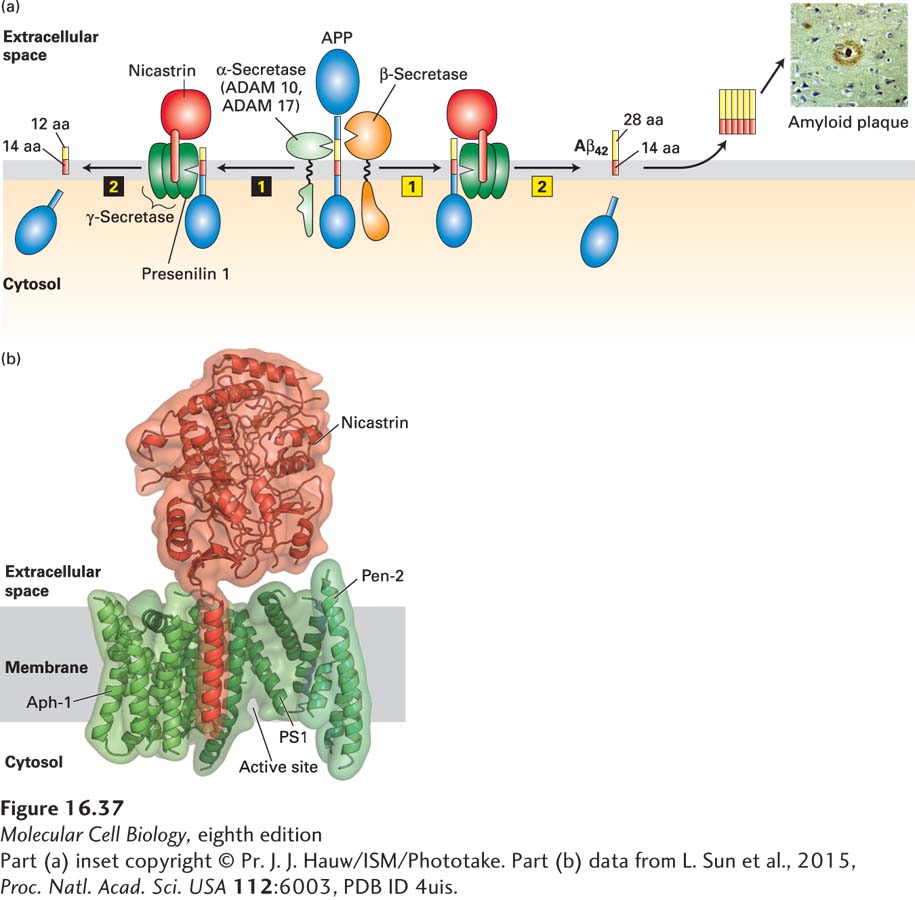
FIGURE 16- 37 Proteolytic cleavage of APP and Alzheimer’s disease. (a, left) Sequential proteolytic cleavage by α-secretase (ADAM 10 or ADAM 17) (step 1) and γ-secretase (step 2) produces an innocuous membrane- embedded peptide of 26 amino acids. (a, right) Cleavage in the extracellular domain by β-secretase (step 1) followed by cleavage within the membrane by γ-secretase (step 2) generates the 42- amino- acid Aβ42 peptide, which spontaneously forms oligomers, and then the large amyloid plaques found in the brains of patients with Alzheimer’s disease (inset). In both pathways, the cytosolic segment of APP is released into the cytosol, but its function is not known. See S. Lichtenthaler and C. Haass, 2004, J. Clin. Invest. 113:1384, and V. Wilquet and B. De Strooper, 2004, Curr. Opin. Neurobiol. 14:582. (b) Three- dimensional structure of human γ-secretase at 0.45 nm resolution. It contains a total of 19 transmembrane segments and a large extracellular domain from nicastrin. The protease catalytic site in PS1 is located near the cytosolic surface. See P. Lu et al., 2014, Nature 512:166.
[Part (a) inset copyright © Pr. J. J. Hauw/ISM/Phototake. Part (b) data from L. Sun et al., 2015, Proc. Natl. Acad. Sci. USA 112:6003, PDB ID 4uis.]
[Leave] [Close]