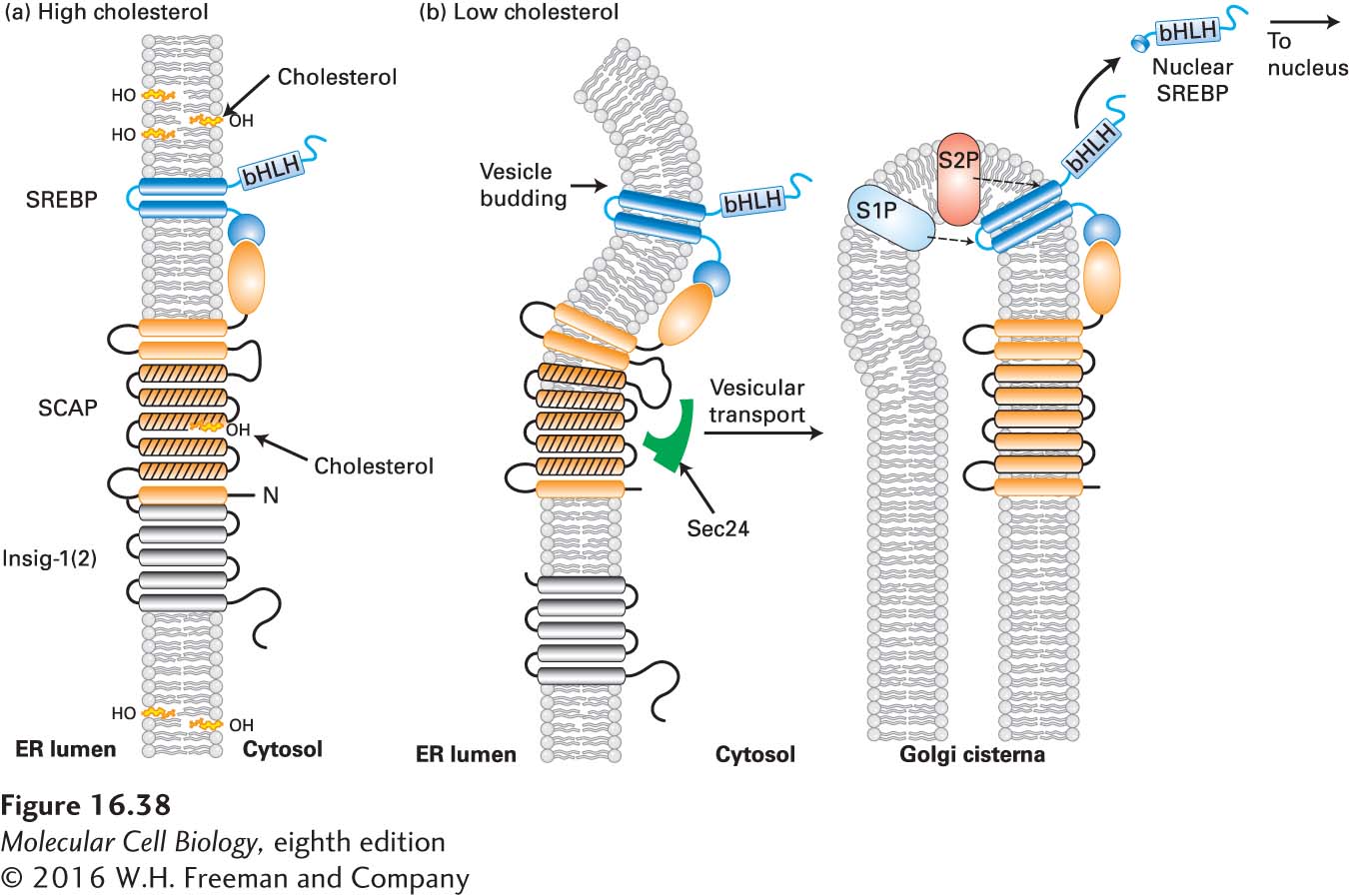
FIGURE 16- 38 Cholesterol- sensitive control of SREBP activation. The cellular pool of cholesterol is monitored by the combined action of insig- 1(2) and SCAP, both transmembrane proteins located in the ER membrane. Membrane- spanning helices 2– 6 of SCAP (orange with black lines) form a sterol- sensing domain, and a C- terminal segment binds to SREBP. (a) When cholesterol levels are high enough that ER cholesterol exceeds 5 percent of total ER lipids, cholesterol binds to the sterol- sensing domain in SCAP, triggering a conformational change that enables the N- terminal SCAP domain to bind to insig- 1(2), anchoring the SCAP– SREBP complex in the ER membrane. (b) At low cholesterol levels, cholesterol dissociates from the SCAP sterol- sensing domain, triggering a reverse conformational change that dissociates SCAP from insig- 1(2) and enables SCAP to bind to Sec24, a subunit of the COPII complex (see Figure 14- 8 ). This binding initiates movement of the SCAP- SREBP complex to the Golgi complex by vesicular transport. In the Golgi, the sequential cleavage of SREBP by the site 1 and site 2 proteases (S1P, S2P) releases the N- terminal bHLH domain of SREBP, which translocates to the nucleus, and SCAP, which recycles to the ER. In the nucleus, the released SREBP domain, called nuclear SREBP (nSREBP), controls the transcription of genes containing sterol regulatory elements (SREs) in their promoters. See A. Radhakrishnan, 2008, Cell Metab. 8:451, and M. Brown and J. Goldstein, 2009, J. Lipid Res. 50:S15.
[Leave] [Close]