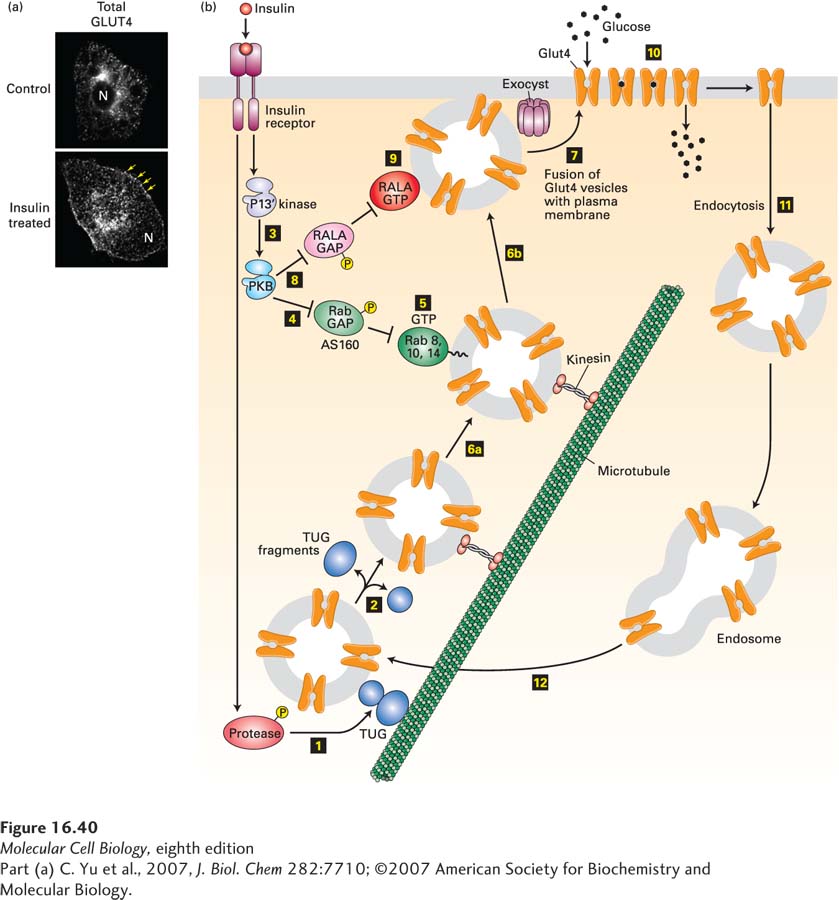
EXPERIMENTAL FIGURE 16- 40 Insulin stimulation of fat cells induces translocation of GLUT4 from intracellular vesicles to the plasma membrane. (a) Cultured adipose cells engineered to express a chimeric protein comprising GLUT4 with a green fluorescent protein (GFP) fused to its C- terminus were visualized with a confocal fluorescence microscope. In the absence of insulin, virtually all of the GLUT4 is in intracellular membranes. Treatment with insulin triggers fusion of the GLUT4- containing membranes with the plasma membrane. Arrows highlight GLUT4 present at the plasma membrane; N indicates the position of the nucleus. (b) In fat and muscle cells, insulin signaling acts in multiple steps to increase the level of GLUT4 at the plasma membrane. In resting cells, the majority of the GLUT4 protein is localized to specialized GLUT4 storage vesicles (GSVs), tethered to Golgi matrix proteins by the TUG protein. Binding of insulin to the insulin receptor leads to activation of a protease (step 1) that cleaves the TUG protein, releasing GLUT4- containing vesicles (step 2), which then move along microtubules, powered by a kinesin motor (see Chapter 18), to the cell surface. Insulin also activates PKB (step 3; see Figure 16- 29 ). PKB then phosphorylates the Rab GAP protein AS160 (step 4), inhibiting its ability to accelerate GTP hydrolysis by Rab8, Rab10, and Rab14. These Rab proteins accumulate in their active GTP- bound states (step 5) and allow the GLUT4 storage vesicles to move along microtubules to the cell surface (steps 6a and 6b). Finally, these GSVs fuse with the plasma membrane (step 7). This step is catalyzed by the exocyst and also by another monomeric GTP- binding protein, RALA. PKB stimulates this membrane fusion event by phosphorylating and thus inactivating the RALA GAP protein RGC (step 8), allowing RALA to accumulate in its active GTP- bound state (step 9). The resultant increase in plasma membrane GLUT4 allows the cell to incorporate glucose from the extracellular fluids at a rate about 10 times that of unstimulated cells (step 10). Following removal of insulin, the plasma membrane GLUT4 is internalized by endocytosis (step 11) and eventually transported to GSVs (step 12). Many other proteins, not shown here, participate in these signaling and vesicle budding and fusion events. See J. Bogan, 2012, Annu. Rev. Biochem. 81:507, D. Leto and A. Saltiel, 2012, Nat. Rev. Mol. Cell Biol. 13:383, and J. Belman et al., 2014, Rev. Endocr. Metab. Disord. 15:55.
[Part (a) C. Yu et al., 2007, J. Biol. Chem 282:7710; ©2007 American Society for Biochemistry and Molecular Biology.]
[Leave] [Close]